ABSTRACT
Introduction: Children with newly diagnosed acute lymphoblastic leukemia (ALL) present with low peripheral blood counts caused by bone marrow replacement. The recovery of counts during induction chemotherapy is not well described.
Material and methods: Records for 63 children with ALL were reviewed. Peripheral hematology blood counts during five weeks of induction chemotherapy were extracted, and the time to partial recovery with safe counts and complete recovery with normal counts in the three cell lines determined. The number of red cell and platelet transfusions, the number of febrile episodes, and the number of days on intravenous antibiotics were counted.
Results: Platelet recovery occurred early: median time to achieving counts >50/nL 14 days, to counts >100/nL 16 days. Neutrophil recovery was relatively slow: median time to counts >0.5/nL 18 days, to counts >1.0/nL 26 days. The time to partial recovery was shorter in high risk than in lower-risk treatment groups. Partial platelet recovery by day 15 indicated early recovery and lower morbidity. Complete platelet recovery day 15 was significantly associated with residual disease <0.1% after four weeks. Lymphocyte counts showed a marked decrease in first two weeks followed by a rise in the next three weeks; a count <0.35/nL on day 15 was associated with poor response.
Conclusion: After starting chemotherapy for ALL, platelet recovery can be expected after two to three weeks while neutrophil recovery lasts three to five weeks. Platelet and lymphocyte counts after two weeks treatment may give an indication of residual disease after four weeks.
Introduction
In Denmark about 40 children are diagnosed with acute lymphoblastic leukemia (ALL) every year, making it the most frequent malignant disease entity in childhood. The disease is characterized by accumulation of lymphoblasts in organs (lymph nodes, liver, spleen) and by infiltration of the bone marrow (BM) with blasts. Because of the marrow displacement erythropoiesis, granulopoiesis and thrombopoiesis are suppressed, and typically all three cell lines are involved with low counts in peripheral blood. The bone marrow failure results in anemia, bleeding and bacterial infection, the classic presenting triad [Citation1].
Initial chemotherapy aims at induction of complete remission, i.e. clearance of blasts from the bone marrow on morphological examination, usually achieved after four weeks of treatment. As leukemia is cleared the normal bone marrow can regenerate, resulting in normal peripheral counts. This process of regeneration is important as a sign of treatment effect, and in regaining normal BM function and recovering from the clinical problems caused by BM insufficiency. The period with very low counts is associated with morbidity, i.e. need for transfusion therapy and antibiotic treatment. Furthermore, this period is associated with a risk of serious and life-threatening complications; thus, there is an induction mortality of 1%, mostly from bacteremia, occasionally from severe bleeding [Citation2].
In the management of children with ALL commencing chemotherapy, knowledge of the expected duration of the risk period and the time to complete BM regeneration would be useful. A rapid recovery obviously reduces morbidity, but also could be assumed to be a sign of good response to treatment. Publications concerning these issues are sparse [Citation3,Citation4]. We have therefore reviewed a cohort of children with newly diagnosed ALL in order to describe the recovery of BM function after initiation of induction chemotherapy, to determine if it differs in the therapeutic risk groups, and to examine if recovery is related to treatment response as assessed by the amount of residual disease after four weeks. In addition, we aimed to describe the changes in lymphocyte counts during induction treatment – possibly an important host factor in tumor immunity [Citation5,Citation6] – and explore their prognostic significance.
Materials and methods
From July 2000 to June 2014, 63 children and adolescents aged 1–18 years were diagnosed with ALL at Aalborg University Hospital. The immunophenotype was precursor B-cell ALL in 57, T-cell ALL in 6. The children were treated according to the Nordic collaborative treatment protocols NOPHO ALL-2000 (n = 32) or NOPHO ALL-2008 (n = 31). They were stratified to standard risk (SR), intermediate risk (IR) or high risk (HR) therapy according to risk factors including leukocyte count, immunophenotype, cytogenetic changes and response to therapy evaluated by minimal residual disease (MRD). Induction chemotherapy consisted of Prednisone for five weeks, six weekly doses of Vincristine, two doses of Doxorubicin, and four doses of intrathecal Metotrexate; for cases with high risk factors at the time of diagnosis a third dose of Doxorubicin was given, and in ALL-2008 Prednisone was replaced with Dexamethasone. Treatment response was assessed by BM examination days 15 and 29. MRD was measured by multicolor flow cytometry for pre-B ALL, with PCR for T-ALL; the criterion for good response was MRD <0.1% at day 29.
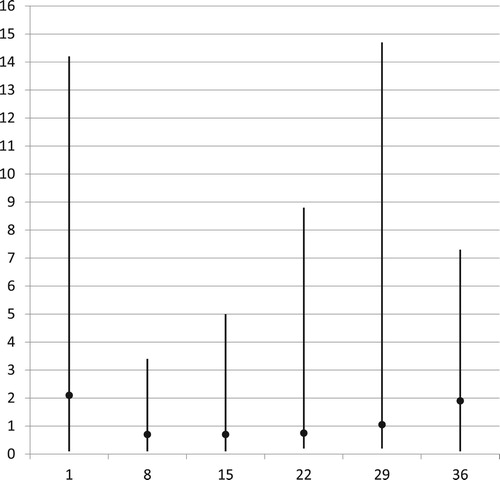
Patient records were reviewed to describe BM regeneration during the induction period as reflected in the rise of peripheral blood counts. The following data were extracted: the date of commencing induction chemotherapy; the dates of measuring the last Hemoglobin (Hb) value <5.0 mmol/L, the last platelet count (PLC) <50/nL, and the last absolute neutrophil count (ANC) <0.5/nL, all subsequent counts remaining above these levels (defining partial recovery); the dates of measuring the last Hb <6.0 mmol/L, PLC <100/nL, and ANC <1.0/nL (complete recovery); BM morphology and MRD at days 15 and 29; and the numbers of erythrocyte transfusions, platelet transfusions, febrile episodes, bacteremic episodes and days with intravenous antibiotics as measures of morbidity associated with poor BM function. In addition, the absolute lymphocyte counts (ALC) on days 1, 8, 15, 22, 29 and 36 were recorded. For brevity, counts at different times have been written with a suffix for treatment day, i.e. ALC-1, ALC-8, etc.
Data were analyzed descriptively. For each case, the duration of the periods to partial and complete recovery of the three cell lines were calculated from the recorded dates. For the whole cohort or for subgroups of children, BM regeneration was visualized in plots showing the percentages having achieved partial or complete recovery. Average times to recovery and measures of morbidity were compared for the three risk groups, and for children with good and poor treatment response.
The study was retrospective and non-interventional, and data were anonymized. Data collection was approved by data registration authorities. Ethical review and informed parental consent was not required.
Results
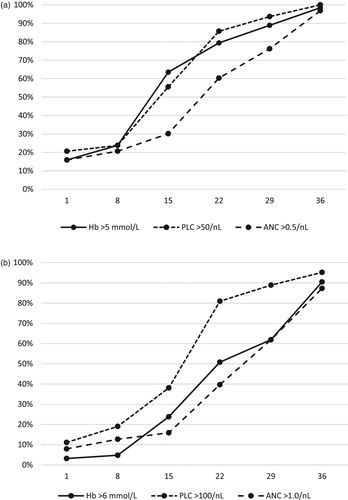
The frequency of severe suppression at diagnosis was 80% for all three counts ((A)). Partial Hb and PLC recovery in most cases occurred in the second and third week of induction, whereas partial ANC recovery occurred more slowly in the third to fifth week. The median time to partial recovery was 13 days for Hb, 14 days for PLC and 18 days for ANC. At day 29, one-quarter of the children still were severely neutropenic.
Figure 1. Recovery of peripheral blood counts during induction chemotherapy treatment days 1–36 in 63 children with ALL. (A) Partial recovery, percentages of children in whom Hb values have stabilized >5.0 mmol/L (solid), platelet counts >50/nL (dotted), and neutrophil counts >0.5/nL (stippled). (B) Complete recovery, percentages with Hb >6.0 mmol/L (solid), platelet counts >100/nL (dotted), and neutrophil counts >1.0/nL (stippled).
The duration of severe cytopenia was similar in the three risk groups, but for all three cell lines shorter in the HR group than in the IR and SR groups (). The morbidity in terms of transfusions and antibiotic therapy was similar in the three groups.
Table 1. Comparison of time to partial recovery from very low blood counts during induction chemotherapy and the associated morbidity in children with standard risk, intermediate risk, and high risk ALL.
Partial platelet recovery by day 15, seen in 56% of children, could be used as a marker of early recovery (). The duration of severe anemia and severe neutropenia was shorter in this group, the need for blood product transfusions considerably lower, and the infectious morbidity lower.
Table 2. Comparison of morbidity during induction in children with early recovery, defined as PLC stabilized >50/nL by day 15, and late recovery. The group with early recovery includes six children with all three counts normal throughout induction.
Complete PLC recovery typically occurred in the third week, while normalization of Hb and ANC generally was delayed, occurring in the third, fourth or fifth week ((B)). Median time to complete recovery was 16 days for PLC, 20 days for Hb and 26 days for ANC.
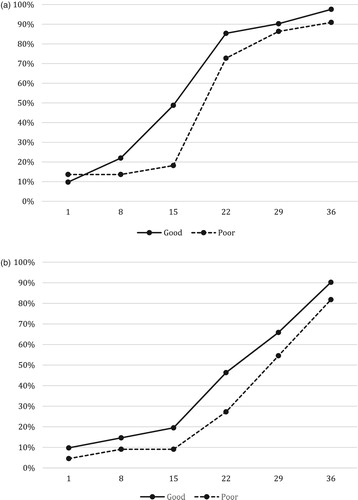
Complete recovery of PLC and ANC tended to occur a little faster in good responders than in poor responders (). PLC-15 had become normal in 49% vs. 18% (OR 4.29, CI 1.23–14.9), i.e. significantly more frequently. ANC-22 had become normal in 46% vs. 27% (OR 2.30, CI 0.75–7.1). These differences were more pronounced on the NOPHO-2000 protocol than on the NOPHO-2008 protocol (data not shown).
Figure 2. Comparison of recovery in children with ALL with good response (MRD <0.1% day 29, n = 41) and with poor response (MRD day 29 ≥ 0.1%, n = 22). (A) Percentages of good (solid) and poor (stippled) responders with platelet count stabilized >100/nL. (B) Percentages of good (solid) and poor (stippled) responders with neutrophil count stabilized >1.0/nL.
The lymphocyte counts showed a characteristic change during induction therapy, with a marked decrease in the first two weeks followed by regeneration over the next two to three weeks (). Median ALC at days 1 – 15 – 29 – 36 were 2.1 – 0.7 – 1.05 – 1.9/nL. The percentages with lymphopenia <1.0/nL at these treatment days were 31% – 68% – 43% – 21%. At day 15, lymphopenia was more frequent in poor responders than in good responders (79% vs. 63%, OR 2.19, CI 0.61–7.91), and severe lymphopenia with ALC < 0.35/nL close to significantly more frequent (42% vs. 18%, OR 3.22, CI 0.95–10.97). At day 36, continuing lymphopenia was seen more often in poor than in good responders (28% vs. 17%, OR 1.86, CI 0.48–7.21).
Discussion
In this study we have described the recovery from peripheral cytopenia after starting induction chemotherapy in a cohort of children with ALL. According to our findings, recovery from transfusion-requiring thrombocytopenia and anemia may be expected after two to three weeks, while recovery from severe neutropenia occurs more slowly after three to five weeks. Once partial recovery has occurred, normalization of the count follows shortly. There was no difference in time to recovery between the three risk groups. Three markers of recovery that may be clinically useful were identified: (1) PLC-15 ≥ 50/nL indicated rapid recovery with low morbidity, (2) PLC-15 ≥ 100/nL was associated with good treatment response, and (3) ALC-15 ≤ 0.35/nL, i.e. a very low lymphocyte nadir, was associated with poor response.
The patient series is complete and consecutive, and the data extracted from the records are reliable with few missing values. Thus, the description of BM recovery is likely to be accurate. The children were treated on two different protocols with differences in risk stratification but with similar induction chemotherapy, and no marked differences in pattern of recovery were seen. The analyses of associations should be interpreted with some caution because of small numbers, but statistically significant associations nonetheless were found. The direct relation of recovery parameters to risk of relapse or to survival has not been explored, the number of treatment failures being too small for meaningful analysis. Instead, we used MRD-29, the most powerful prognostic factor, as a surrogate marker.
To our knowledge, recovery of the three cell lines in the BM during induction chemotherapy has not previously been reported in the literature. We found a characteristic sequence with relatively rapid PLC regeneration followed shortly by Hb recovery, while neutrophil regeneration was somewhat slow. In terms of morbidity, partial recovery is most important. From the pattern of recovery it appears that bleeding or anemia requiring transfusion therapy mainly occurs in the first two weeks of induction, while the risk of infection or bacteremia often persists throughout induction. Once partial recovery of a cell line has occurred, complete recovery can be expected in a weeks time.
Contrary to expectation, we found that the period with severely suppressed counts on average was shorter in HR children than in IR or SR children. The need for transfusions and antibiotics was similar in the three groups, however, meaning that supportive interventions were delivered at a higher rate in HR children. Thus, the period may be shorter but more eventful. Possibly this may be ascribed to the use of dexamethasone rather than prednisone.
PLC-15 ≥ 50/nL was seen in more than half the children and was a marker of early marrow recovery with shorter duration of severe anemia and severe neutropenia. As a consequence, the morbidity in terms of need for blood products and antibiotic therapy was lower in this group. Thus, PLC-15 > 50/nL may be a useful harbinger of impending BM regeneration, indicating that protracted induction morbidity is unlikely.
Rapid bone marrow recovery is advantageous in terms of morbidity, but according to recent evidence also may be a favorable prognostic factor. In adults with ALL, shorter time to platelet recovery has been associated with better disease free survival and overall survival [Citation7], possibly reflecting host ability to overcome MRD [Citation8]. In children, a high PLC at end of induction (upper quartile) was significantly associated with better outcome [Citation4]. In addition, a low ANC at end of induction (below median) was associated with more relapses than expected, independent of the amount of MRD [Citation3]. In our small cohort, it is not possible to analyse the relation between BM recovery and outcome. We did find, however, that complete recovery of PLC-15 was predictive of low MRD-29, which is a favorable prognostic factor. This indirectly supports a prognostic role for BM recovery.
In recent years, several reports have shown that the lymphocyte counts during induction also have a prognostic significance. Most children show a striking pattern with development of lymphopenia by mid-induction followed by lymphocyte recovery by the end of induction. In 2008, De Angulo et al. [Citation9] reported that a very low lymphocyte nadir, ALC-15 < 0.35/nL, was a strong and independent predictor of a high event rate in children and adolescents with ALL and with AML. Since then, two Chinese studies using ratios of mid-induction ALC to initial ALC have shown that a low ratio is correlated with high MRD [Citation5] and associated with an inferior event free survival [Citation6]. In our study we found that ALC-15 < 0.35/nL was twice as frequent among poor than among good responders. Thus, a modest fall in ALC may somehow reflect robustness of the host.
The subsequent recovery of lymphocytes also may have prognostic value, with low counts at end of induction being an adverse factor. Studies have shown that ALC-29 < 0.35/nL, is associated with poor survival [Citation10], that ALC > 0.5/nL at end of induction indicates better survival in MRD-negative children [Citation11], and that ALC-29 > 1.5/nL indicates better relapse free survival in both MRD-positive and MRD-negative patients [Citation12]. The latter finding, however, could not be replicated in a subsequent study in an ethnically different group treated on a seven-drug induction [Citation13]. In our study, there was no difference in ALC-29 between good and poor responders. Further studies including subset analyses are needed to elucidate whether persisting lymphopenia might indicate a lack of anti-tumor cells.
In conclusion, this retrospective study has shown that the three cell lines regenerate at different rates as induction chemotherapy clears the BM of leukemia cells, with rapid platelet recovery and slow neutrophil recovery. PLC-15 may be a useful clinical marker, partial recovery indicating early BM regeneration with low induction morbidity, and complete recovery increasing the probability of good response to chemotherapy. In addition, our study confirms recent evidence that changes in ALC during induction may be prognostically significant, with a very low nadir at day 15 pointing to poor treatment response. Further studies are needed to investigate whether the changes in peripheral counts during induction may have a role in treatment stratification.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Brix N, Rosthøj S. Bone marrow involvement is not manifest in the early stages of childhood acute lymphoblastic leukemia. Dan Med J. 2014;61(8):A4883.
- Lund B, Åsberg A, Heyman M, et al. Risk factors for treatment related mortality in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(4):551–559. doi: 10.1002/pbc.22719
- Laughton SJ, Ashton LJ, Kwan E, et al. Early responses to chemotherapy of normal and malignant hematologic cells are prognostic in children with acute lymphoblastic leukemia. J Clin Oncol. 2005;23(19):2264–2271. doi: 10.1200/JCO.2005.04.012
- Zeidler L, Zimmermann M, Möricke A, et al. Low platelet counts after induction therapy for childhood acute lymphoblastic leukemia are strongly associated with poor early response to treatment as measured by minimal residual disease and are prognostic for treatment outcome. Haematologica. 2012;97(3):402–409. doi: 10.3324/haematol.2011.045229
- Shen HQ, Feng JH, Tang YM, et al. Absolute lymphocyte count is associated with minimal residual disease level in childhood B-precursor acute lymphoblastic leukemia. Leukemia Res. 2013;37(6):671–674. doi: 10.1016/j.leukres.2013.02.002
- Cheng Y, Luo Z, Yang S, et al. The ratio of absolute lymphocyte count at interim of therapy to absolute lymphocyte count at diagnosis predicts survival in childhood B-lineage acute lymphoblastic leukemia. Leukemia Res. 2015;39(2):144–150. doi: 10.1016/j.leukres.2014.11.013
- Faderl S, Thall PF, Kantarjian HM, et al. Time to platelet recovery predicts outcome of patients with de novo acute lymphoblastic leukemia who have achieved a complete remission. Br J Haematol. 2002;117(4):869–874. doi: 10.1046/j.1365-2141.2002.03506.x
- Faderl S, Estrov Z. Hematopoietic recovery following induction therapy of acute leukemias: prognostic implications and a new look at the definition of remission. Leuk Lymphoma. 2004;45(1):67–71. doi: 10.1080/1042819031000151914
- De Angulo G, Yuen C, Palla SL, et al. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML. Cancer. 2008;112(2):407–415. doi: 10.1002/cncr.23168
- Hatzipantelis E, Pana ZD, Vlachou M, et al. Peripheral blood lymphocyte recovery and overall survival in pediatric acute lymphoblastic leukemia (letter). Pediatr Blood Cancer. 2014;61(1):181–183. doi: 10.1002/pbc.24736
- Rubnitz JE, Campbell P, Zhou Y, et al. Prognostic impact of absolute lymphocyte counts at the end of remission induction in childhood acute lymphoblastic leukemia. Cancer. 2013;119(11):2061–2066. doi: 10.1002/cncr.28026
- Rabin KR, Gramatges MM, Borowitz MJ, et al. Absolute lymphocyte counts refine minimal residual disease-based risk stratification in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59(3):468–474. doi: 10.1002/pbc.23395
- Alkayed K, Halalsheh H, Khattab E, et al. Lack of prognostic significance of absolute lymphocyte count after intensive induction therapy in childhood acute lymphoblastic leukemia (letter). Pediatr Blood Cancer. 2012;59(2):351. doi: 10.1002/pbc.24120