ABSTRACT
Objectives: To evaluate the prognosis of adult severe aplastic anemia (SAA) patients with absolute neutrophil count (ANC) values of zero prior to immunosuppressive therapy (IST).
Methods: Patients with ANC values of zero prior to IST were separated from very SAA and analyzed in a prospective study. All patients received IST with rabbit anti-thymocyte globulin (ATG) and cyclosporine (CsA).
Results: A significantly lower response rate (RR) was identified in patients with ANC = 0 prior to IST when compared to patients with SAA after both 3 and 6 month periods or compared to those with vSAA at 3 months only. The efficacy of IST was inversely related to ANC = 0. The overall survival rate of the ‘zero’ group was significantly lower than that of the vSAA or SAA groups. Overall survival was closely associated with response to IST, and was inversely related to ANC = 0.
Discussion: In SAA patients, ANC is associated with prognosis, the elucidated overall survival improvement in patients without ANC = 0 occurred in conjunction with decreased infection-related mortality. Our study revealed that adult patients with ANC = 0 prior to IST responded poorly to IST, suggesting that having a very low number of neutrophils was a highly predictive factor for efficacy and survival of SAA patients treated with IST.
Conclusion: Adult SAA patients with ANC = 0 had a very poor prognosis and new therapeutic regimens may result in better outcome for these patients.
1. Introduction
Severe aplastic anemia (SAA) can be successfully treated with allogeneic hematopoietic stem cell transplantation (HSCT) from an HLA-matched sibling donor. Intensive immunosuppressive therapy (IST) consisting of anti-thymocyte globulin (ATG) and cyclosporine (CsA) is often used for patients with SAA who lack an HLA-matched sibling donor or who are not eligible for HSCT [Citation1–5].
Early experiments showed absolute neutrophil count (ANC) was highly predictive of response and survival in SAA patients treated with IST. In a report by the European Group for Blood and Marrow Transplantation (EBMT), the actuarial 5-year survival rates were 46% in patients with an ANC of less than 0.2×109/L and 61% in patients with an ANC between 0.2 and 0.5×109/L, here the only significant pre-treatment variables were a low neutrophil count (p = 0.001) and increasing age (p = 0.05) [Citation6]. A study analyzed 64 patients with aplastic anemia treated with antilymphocyte globulin between 1980 and 1985, and revealed 79% survival for non-severe aplastic anemia (NSAA), compared to 36% for SAA (p = 0.001). The neutrophil and platelet counts before treatment with ALG were highly predictive of survival [Citation7]. A randomized trial from the EBMT SAA working party demonstrated that early mortality (< 120 d) was correlated with the severity of disease (39%, 10% and 6% respectively in patients with neutrophil counts of < 0.2, 0.2–0.5, > 0.5×109/L) [Citation8]. Bacigalupo et al. further proposed that AA was considered very severe if the criteria for SAA were fulfilled and the neutrophil count was less than 0.2×109/L. SAA was subclassified as very SAA (vSAA) (ANC of <0.2×109/L) and SAA (ANC of 0.2–0.5×109/L).
But recently, one study in children showed that younger age, higher baseline absolute reticulocyte count (ARC), and absolute lymphocyte count (ALC) were highly predictive of response to IST at 6 months (p = 0.018, p = 0.005 and 0.036, respectively) while ANC was not [Citation9]. Another study even reported that patients with a white blood cell count (WBC) of less than 2.0×109/L showed a higher response rate than those with a WBC of 2.0×109/L or more (p = 0.0003). Shorter intervals between diagnosis and therapy (p = 0.01) and being of male sex (p = 0.03) were also associated with a higher response rate [Citation10].
It may be controversial to suggest that the severity of the disease or neutrophil counts at diagnosis can predict the prognosis of SAA after IST.
A prospective Japanese study in children with SAA proposed further subclassification of SAA into fulminant aplastic anemia (FAA), if ANC = 0 for at least 2 weeks prior to and after IST. They found that the response rate (RR) of patients with the FAA was significantly lower than those with vSAA and SAA, but, overall survival (OS) was similar [Citation11]. Does that mean ANC = 0 might be an effective prognostic factor to predict the efficacy of IST?
We retrospectively analyzed the efficacy of IST for adult patients with SAA who were enrolled in a prospective multi-center registration of the Chinese Eastern Collaboration Group of Anemia and evaluated whether the prognosis was impacted by ANC = 0 prior to IST.
2. Patients and methods
2.1. Patients
From January 2014 to March 2018, a total of 91 patients aged 18 years and over with vSAA or SAA in the Hospital A B and C were enrolled in this study.
A diagnosis of SAA was given if at least two of the following peripheral blood count criteria were fulfilled: An ANC of <0.5×109/L, a reticulocyte count of <20×109/L, or platelet count of <20×109/L with a bone marrow cellularity of less than 25% [Citation12].
A vSAA was diagnosis was applied if the criteria for SAA were fulfilled and a patient’s ANC was less than 0.2×109/L [Citation8]. Patients with ANC values of zero were separated from vSAA and identified as the ‘zero’ group in our study.
Bone marrow biopsy and aspiration, for morphology and cytogenetics were performed on all patients. The presence of a paroxysmal nocturnal hemoglobinuria (PNH) clone using flow cytometry was defined as the absence of glycosylphosphatidylinositol (GPI)-anchored surface proteins greater than 1% of neutrophils or monocytes in peripheral blood.
2.2. Treatment regimens
All patients were treated with a combination of intravenous rabbit ATG (Lymphoglobulin, Sanofi, FR) at 3.5 mg/kg/day for 5 days and oral CsA at 6 mg/kg/day. The dose of CsA was adjusted to a trough between 150 and 200 ng/ml for at least 6 months. Granulocyte colony-stimulating factor was administered subcutaneously at 200 μg/m2 only to patients with an ANC of <0.2×109/L.
2.3 Criteria for response to IST
The response was evaluated at 3 and 6 months after the initiation of therapy. A complete remission (CR) was defined as a hemoglobin level of more than 11.0 g/dL, a neutrophil count of more than 1.5×109/L, and a platelet count of more than 150×109 /L. A partial remission (PR) was defined as a patient being transfusion independent and no longer meeting criteria for severe disease. None remission (NR) was defined as a patient still fulfilling the severe disease criteria. The overall response rate (RR) was defined as CR or PR at 3 and 6 months after IST [Citation1].
2.4. Statistical analysis
Data was analyzed using SPSS 20.0. Differences among variables were evaluated by the χ2 test (or Fisher’s exact test for cell frequencies less than 5) and a standard t-test was used for continuous variables. Correlation analysis of relevant factors and efficacy or death were analyzed by the log-rank test. Overall survival was estimated by using the Kaplan-Meier method. P values of less than 5% were considered statistically significant.
3. Results
3.1. Baseline characteristics
Patients were classified into the following three groups by neutrophil stratification: 20 with an ANC of ‘zero’, 26with vSAA, and 45 with SAA. Eleven patients had PNH clones at the time of diagnoses. The median interval between diagnosis and treatment was 9 (3–38), 15 (3–51), and 13 (3–97) days, respectively. The median follow-up at the time of analysis was 17.0 (1–51), 17.5 (4–41), and 27.5 (3–47) months, respectively. There were no significant differences elucidated between baseline characteristics when comparing groups ().
Table 1. Patients’ characteristics.
2.2. Treatment response
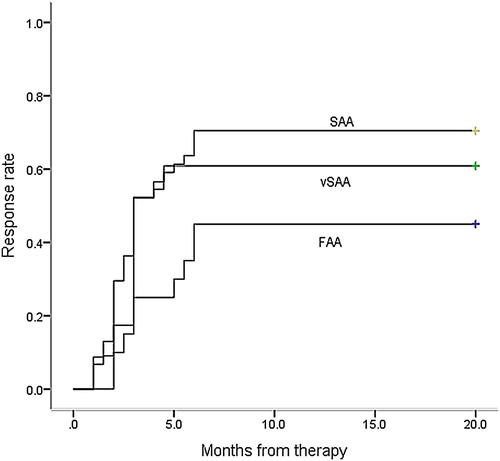
At 3 months after the initiation of therapy, 25 patients (55.6%) with SAA, 14 patients (53.8%) with vSAA, and 5 patients (25.0%) in the ‘zero’ group responded to the initial course of IST (). By 6 months, 33 patients (73.3%) with SAA, 15patients (57.7%) with vSAA, and 9 patients (45.0%) with ‘zero’ group had become transfusion-independent. Six months later, 2 patients achieved CR in the ‘zero’ group, meanwhile, 3 patients achieved PR and6 patients achieved CR in the SAA group. Overall, of the 91 evaluable patients who received an initial course of IST, 11 patients (12.1%) showed complete remission and 46 patients (50.5%) showed partial remission, resulting in an overall response rate of 62.6% after 6 months.
Table 2. Hematologic response in patients treated with immunosuppression.
There was no difference between the remission rates after IST when comparing the SAA and vSAA groups at 3 months (55.6% vs 53.8%, p = 0.934), or 6 months (73.3% vs 57.7%, p = 0.33). However, a significantly lower RR to IST was observed in patients in the ‘zero’ group, when compared to patients in the SAA and vSAA groups at 3months (25.0% vs 55.6%, p = 0.024 and 25.0% vs 53.8%, p = 0.036, respectively). When the 6-month RR to IST was analyzed separately, the ‘zero’ group showed a similar RR to the vSAA group (45.0% vs 57.7%, p = 0.298), but had a significantly poorer RR than the SAA group (45.0% vs 73.3%, p = 0.031).
shows remission to IST, using the Kaplan-Meier method, estimated for all patients in the SAA, vSAA, or ’zero’ groups. Both the SAA and vSAA groups achieved remission faster than the ‘zero’ group did (p = 0.001, p = 0.001) (). No significant difference was identified described between the vSAA and SAA groups (p = 0.420).
3.3. Influencing factors of efficacy
Univariate analysis revealed that IST efficacy was related to the neutrophil count (p = 0.029), but was not related to age (p = 0.599), gender (p = 0.195), time from diagnosis to treatment (p = 0.195), lymphocyte count (p = 0.723), reticulocyte count (p = 0.285), platelet count (p = 0.963), or PNH clone presences (p = 0.256). Furthermore, the neutrophil count was the only factor shown to influence treatment efficacy when using multivariate analysis with a log-rank test (p = 0.019, ).
Table 3. Influence factors of IST efficacy for AA patients.
3.4. Survival and outcomes
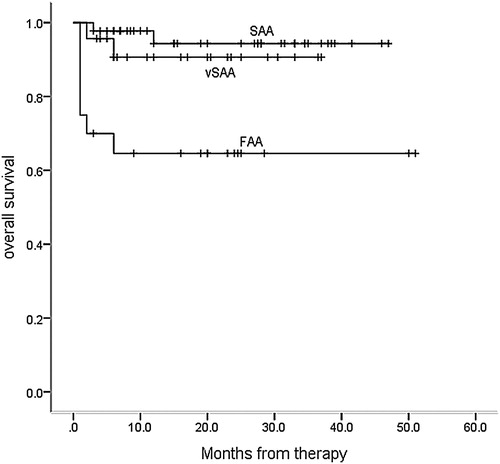
Overall survival in the ‘zero’, vSAA, and SAA groups were 65.0% (95%CI, 39.0–85.5%), 91.7% (95%CI, 75.2–99.9%), and 95.6% (95%CI, 89.0–99.9%) (p = 0.001), respectively ().
Figure 2. The overall survival (OS) of patients in the ‘zero’ group was significantly lower than those in vSAA and SAA groups (p = 0.001) (Kaplan-Meyer method).
There were 7, 2, and 2 patients that died in the ‘zero’, vSAA, and SAA groups respectively. The deaths were due to the following causes: infection (n = 6), respiratory failure (n = 1), deadly hemoptysis (n = 1), intracranial hemorrhage (n = 1), infection with hemorrhage (n = 1), and other reason (n = 1). Among the 6 patients that died, there were 4, 1, and 1 from the ‘zero’, vSAA, and SAA groups respectively. Eight patients dropped out of IST: 5 patients had clonal evolution (4 PNH and 1 MDS-EB-1), and 3 patients needed SCT.
When analyzing factors predictive of OS, including neutrophil count, IST efficacy, age, gender, time from diagnosis to treatment, lymphocyte count, reticulocyte count, platelet count and presence of PNH clones, only neutrophil count and IST efficacy were found to be predictive of improved survival when the multivariate analysis was applied (p = 0.026, p = 0.010) ().
Table 4. Risk factors of death for AA patients after IST.
4. Discussion
Aplastic anemia (AA) is characterized by peripheral blood pancytopenia and classified according to the severity of cytopenia in clinic. In SAA patients a low ANC is associated with poor prognosis, therefore, patients with ANC < 0.2×109/L are further defined as vSAA, indicating increased disease severity [Citation8]. Some researches confirmed that patients might meet a higher incidence of fatal infection and a lower survival rate after ATG treatment with a low ANC [Citation6, Citation12].
The influences of neutrophils on prognosis are various and there are some important research results from several studies in children. A Japanese study showed that at 3 months after the initiation of therapy, 58% patients with SAA and 39% patients with vSAA responded to the initial course of IST. After 6 months, 66% patients with SAA and 72% patients with vSAA had evidence of a trilineage response and became transfusion-independent [Citation13]. A German pediatric study even showed a significantly better CR and survival rate in response to IST in patients with vSAA compared to those with SAA (CR 69% vs 44%, respectively, P = 0.004) [Citation14].
The NIH group investigated the clinical outcomes of 420 patients with SAA over two decades, they showed that the overall 5-year patient survival level improved in conjunction with a decrease in infection-related mortality and frequency of invasive fungal infections (IFIs), as well as in the progression support treatment. When considering the reason for which the comparison of vSAA and SAA groups did not show significant differences in many studies, we postulate that more severe SAA could lead to fatal infection before any response to IST. However, importantly, this research did still show that absolute neutrophil count before IST was associated with prognosis [Citation15].
ANC fell to zero in some of the patients with AA in a Japanese study of SAA in children. The study proposed the concept of FAA and revealed lower RR when comparing patients with vSAA and SAA [Citation11], however, there was no difference in survival rate. Adult SAA patients often have different prognoses to children, since the efficacy of IST is age-related [Citation1]. Therefore, it is necessary to analyze the results of IST in adult patients with SAA and an ANC of zero specifically.
In our study, patients with an ANC of zero took longer to achieve remission and had a lower response and survival rate compared with those in the vSAA or SAA group. Infection was the main cause of death (72.7%, 8/11), which was a frequent occurrence in the ‘zero’ group. In the analyses of risk factors related to survival, both univariate and multivariate analyses showed that neutrophil count and IST efficacy were associated with survival.
Additionally, the favorable OS in the SAA patient population may be due to the younger age of this cohort [Citation16,Citation17]. However, a correlation between age and efficacy and OS in adults was not identified in our study. The presence of PNH clones was associated with a good response to IST and better prognosis [Citation18–21]. However, there are also studies in children showing the response rate to IST was higher in patients without PNH clones [Citation22]. These discrepancies were probably caused by the biological differences between adult and paediatric AA. Our analysis also did not find any correlation between PNH clones and the results of IST, as well as risk factors affecting survival. Other possible factors including gender, time from diagnosis to treatment, lymphocyte count, reticulocyte count, platelet count [Citation3] were not shown to be prognosis predictors in our study.
In summary, this study revealed that adult patients with ANC values of zero poorly responded to IST, suggesting that very low neutrophils count, especially 0, was a highly predictive factor for efficacy and survival of SAA patients treated with IST.
Studies on patients with AA and very low neutrophils counts, especially those with zero neutrophils are still rare. Other treatments such as allogeneic hematopoietic stem cell transplantation, or IST combined with eltrombopag require improvements in efficacy, and could lead to better long-term survival for patients with an ANC of zero. Longer follow-up is also needed to determine the effect of eltrombopag, if any, on efficacy and OS in patients with a zero ANC.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Guang-Sheng He http://orcid.org/0000-0002-3944-5897
Additional information
Funding
References
- Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187–207. doi: 10.1111/bjh.13853
- Young NS. Current concepts in the pathophysiology and treatment of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2013;1:76–81. doi: 10.1182/asheducation-2013.1.76
- Peffault de Latour R, Tabrizi R, Marcais A, et al. Nationwide survey on the use of horse antithymocyte globulins (ATGAM) in patients with acquired aplastic anemia: a report on behalf of the French reference center for aplastic anemia. Am J Hematol. 2018;93(5):635–642. doi: 10.1002/ajh.25050
- Boddu P, Garcia-Manero G, Ravandi F, et al. Clinical outcomes in adult patients with aplastic anemia: a single institution experience. Am J Hematol. 2017;92(12):1295–1302. doi: 10.1002/ajh.24897
- Liu C, Shao Z. Aplastic anemia in China. J Transl Int Med. 2018;6(3):134–137. doi: 10.2478/jtim-2018-0028
- Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177–182. doi: 10.1111/j.1365-2141.1988.tb02460.x
- Marsh JC, Hows JM, Bryett KA, et al. Survival after antilymphocyte globulin therapy for aplastic anemia depends on disease severity. Blood. 1987;70:1046–1052.
- Bacigalupo A, Chaple M, Hows J, et al. Treatment of aplastic anaemia (AA) with antilymphocyte globulin (ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA working party. Br J Haematol. 1993;83:145–151. doi: 10.1111/j.1365-2141.1993.tb04645.x
- Scheinberg P, Wu CO, Nunez O, et al. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144:206–216. doi: 10.1111/j.1365-2141.2008.07450.x
- Yoshida N, Yagasaki H, Hama A, et al. Predicting response to immunosuppressive therapy in childhood aplastic anemia. Haematologica. 2011;96:771–774. doi: 10.3324/haematol.2010.032805
- Yagasaki H, Shichino H, Ohara A, et al. Immunosuppressive therapy with horse anti-thymocyte globulin and cyclosporine as treatment for fulminant aplastic anemia in children. Ann Hematol. 2014;93:747–752. doi: 10.1007/s00277-013-1984-x
- Camitta BM, Thomas ED, Nathan DG, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53:504–514.
- Kosaka Y, Yagasaki H, Sano K, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. 2007;111:1054–1059. doi: 10.1182/blood-2007-08-099168
- Führer M, Rampf U, Baumann I, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106:2102–2104. doi: 10.1182/blood-2005-03-0874
- Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52(6):726–735. doi: 10.1093/cid/ciq245
- Tichelli A, Marsh JCW. Treatment of aplastic anaemia in elderly patients aged >60 years. Bone Marrow Transplant. 2013;48(2):180–182. doi: 10.1038/bmt.2012.224
- Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA working party of the European group for blood and marrow transplantation. Blood. 2011;117:4434–4441. doi: 10.1182/blood-2010-08-304071
- Maciejewski JP, Rivera C, Kook H, et al. Relationship between bone marrow failure syndromes and the presence of glycophosphatidyl inositol-anchored protein deficient clones. Br J Haematol. 2001;115:1015–1022. doi: 10.1046/j.1365-2141.2001.03191.x
- Sugimori C, Chuhjo T, Feng X, et al. Minor populations of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anaemia. Blood. 2006;107:1308–1314. doi: 10.1182/blood-2005-06-2485
- Afable MGII, Shaik M, Sugimoto Y, et al. Efficacy of rabbit anti-thymocyte globulin in severe aplastic anemia. Haematologica. 2011;96:1269–1275. doi: 10.3324/haematol.2011.042622
- Yamazaki H, Saito C, Sugimori N, et al. Thymoglobuline is as effective as lymphoglobuline in Japanese patients with aplastic anemia possessing increased glycosylphosphatidylinositol-anchored protein (GPI-AP) deficient cells. Blood (ASH Annual Meeting Abstracts). 2011;118:1339.
- Timeus F, Crescenzio N, Lorenzati A, et al. Paroxysmal nocturnal haemoglobinuria clones in children with acquired aplastic anaemia: a prospective single centre study. Br J Haematol. 2010;150:480–497.