ABSTRACT
Objectives:
The addition of lenalidomide (LEN) to azacitidine (AZA) may further improve the outcomes of acute myeloid leukemia (AML) patients as well as patients with high-risk myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) patients although the evidence for this combination treatment is still relatively limited. This meta-analysis aimed to evaluate efficacy and adverse effects of AZA plus LEN for the treatment of patients with high-risk MDS, AML or CMML.
Methods:
The current study systematically identified all cohort studies of patients with AML and/or MDS and/or CMML who received AZA in combination with LEN that reported the overall complete remission (CR) rate and/or overall response rate (ORR). A DerSimonian–d random–effects model with double arcsine transformation was used for the pooled rates and 95% confidence interval (CI) of the all outcomes.
Results:
A total of 10 studies with 406 patients were identified and included into the meta-analysis. The pooled CR rate after the treatment with AZA-plus-LEN regimen was 33.0% (95% CI, 27.7%–38.7%, I2 = 18%) while the pooled ORR was 49.9% (95% CI, 38.4%–61.5%, I2 = 72%). Nonetheless, adverse events including grade 3–4 neutrophil toxicity events, platelet toxicity events and febrile neutropenia were common with AZA-plus-LEN regimen.
Conclusions:
The current study may serve as a preliminary data to suggest that the addition of LEN may offer incremental benefit to patients with high-risk MDS, AML and CMML. However, randomized-controlled studies that directly compare the efficacy and adverse events of AZA-plus-LEN regimen versus AZA monotherapy are still needed.
Introduction
Acute myeloid leukemia (AML) is a common subtype of leukemia with the median age of onset of approximately 70 years [Citation1]. Prognosis of AML is relatively unfavorable with the reported median overall survival (OS) time of only 9.2 months and 5/year overall survival rate of only 13.5% [Citation2]. Poor prognostic factors for survival include age of over 75 years, poor performance status, high/risk cytogenetics, previous hematologic diseases and treatment with less intensive therapy [Citation3,Citation4]. The National Comprehensive Cancer Network (NCCN) recommends hypomethylating agents (HMAs), such as decitabine and azacitidine (AZA), as the preferred treatment options for elderly AML patients who have unfavorable cytogenetics, poor molecular markers, a history of antecedent hematologic disorder, therapy/related AML or unfit performance status [Citation5]. These medications are also recommended for a patient with high/risk myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) [Citation6]. The recommendations are based on studies that showed a better OS associated with treatment with HMAs compared with other treatment options for elderly patients with AML, including low/dose cytosine arabinoside and intensive chemotherapy [Citation7]. A study on AZA for treatment of high/risk MDS and CMML also found a fairly favorable median OS time of 21.5 months [Citation8].
Lenalidomide (LEN) is an immunomodulatory drug that has been approved for the treatment of a specific subtype of MDS (patients with deletion (del) (5q) cytogenetic abnormalities) [Citation9]. The addition of LEN to AZA may help improving the outcomes of patients with AML, MDS and CMML. However, the evidence for the efficacy of this combination treatment is still relatively limited as the available published studies are generally small in size, yielding considerably varied results [Citation10–19]. This systematic review and meta/analysis was conducted with the aims to identify all studies that used the combination therapy of AZA and LEN for the treatment of patients with high/risk MDS, AML or CMML and summarize their results together in order to comprehensively evaluate its efficacy and adverse effects.
Methods
Data sources and searches
Three investigators (C.K., P.T. and W.O.) independently searched for studies published before September 2018 in MEDLINE and EMBASE database. The search strategy consisted of search terms for acute myeloid leukemia, myelodysplastic syndrome, chronic myelomonocytic leukemia, azacitidine and lenalidomide (Supplementary Data 1). The references of the eligible articles were also reviewed to identify additional studies. The guideline for processing this systematic review and meta/analysis (Preferred Reporting Items for Systematic Reviews and Meta/Analyses) is available as Supplementary Data 2 [Citation20].
Selection criteria and data extraction
Studies were considered eligible for inclusion into this meta/analysis if they were (1) cohort studies (either prospective or retrospective) of patients with AML and/or MDS and/or CMML who received AZA in combination with LEN that (2) reported the primary outcomes of interest (overall complete remission [CR] rate and/or overall response rate [ORR]). The secondary outcomes of interest were complete cytogenetic response rate, disease progression rate, grade 3–4 neutrophil toxicity rate, grade 3–4 platelet toxicity rate, febrile neutropenia rate, thrombotic events rate, acute renal failure rate and treatment/related mortality (TRM) rate. Secondary outcomes were extracted from each study and combined together when available but they were not part of the inclusion criteria. Evaluation of eligibility was independently performed by the three investigators. If different assessments were made regarding the eligibility of the study, the study in question was jointly reviewed by the three investigators and the final determination was reached by consensus.
Definition of treatment response and outcomes
A CR was defined as the presence of all of the following:a bone marrow blast count of < 5%; the absence of circulating blasts and blasts with Auer rods; the absence of an extramedullary disease; a hemoglobin level of ≥11 g/dL; an absolute neutrophil count of ≥1.0 × 109/L; and a platelet count of ≥100 × 109/L. A CR with incomplete recovery of peripheral blood counts (CRi) was defined as meeting the CR criteria except that the peripheral blood counts were not fully restored. A partial remission was defined as a bone marrow blast count which declined by at least 50% relative to the pretherapy level but still remained above 5% [Citation21]. The overall CR rate was the proportion of patients who achieved CR or CRi after therapy; similarly, the ORR was defined as the proportion of patients who achieved CR, CRi or partial remission after therapy. A complete cytogenetic response was defined as disappearance of cytogenetic abnormality [Citation21]. A progressive disease was defined as an increase in percent of blast comparing to the prior treatment level [Citation21]. TRM was defined as death during treatment. Grade 3–4 neutrophil toxicity, grade 3–4 platelet toxicity, febrile neutropenia, thrombosis and acute renal failure were defined according to common terminology criteria for adverse events [Citation22].
Statistical analysis
All data analyses were performed using the Comprehensive Meta/Analysis program, version 2.2 (Biostat, Englewood, NJ, United States) and Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Three authors (C.K., P.T. and W.O.) extracted the data from each study by using a pre/defined data extraction form. A DerSimonian–Laird random/effects model with double arcsine transformation was used for the pooled rates and 95% confidence interval of the overall CR rate, ORR, complete cytogenetic response rate, disease progression rate, rate of grade 3–4 neutrophil toxicity, rate of grade 3–4 platelet toxicity, febrile neutropenia rate, thrombotic events rate, acute renal failure rate and TRM rate [Citation23]. We used the random/effect model, rather than the fixed/effect model, because the included studies were not conducted under the exactly same condition, which would invalidate the assumption of the fixed/effect model. The between/study heterogeneity was assessed using Cochran's Q test and the I2 statistic. The I2/values were classified as follows:0–25% indicated insignificant heterogeneity; 26–50%, low heterogeneity; 51–75%, moderate heterogeneity; and > 75%, high heterogeneity [Citation24].
Results
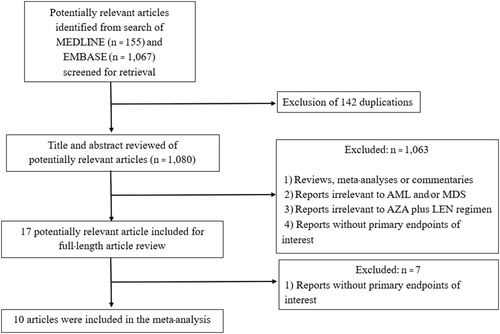
The search strategy yielded 1222 relevant articles (155 from MEDLINE and 1067 from EMBASE). After the exclusion of 142 duplicated articles using EndNote X8 software, 1080 underwent a title and abstract review. A total of 1063 articles were excluded at this stage because they did not meet the inclusion criteria based on the type of article, study design, subjects and/or interventions. Seventeen articles were full/text reviewed and seven of them were excluded on the basis that they did not report the primary outcomes of interest. The 10 remaining studies (eight prospective cohort studies, one retro/prospective cohort study and one retrospective study) fulfilled the eligibility criteria and were included in the meta/analysis [Citation10–19]. demonstrates the literature review and identification process.
Baseline patient characteristics
The meta/analysis evaluated the efficacy and toxicity of the AZA/plus/LEN regimen for a total of 406 patients with AML, MDS and CMML across 10 cohort studies [Citation10–19]. The age of the patients ranged from 23 to 88 years, with a male predominance (59.3%) [Citation10–19]. MDS was the most common diagnosis (55.3%), followed by AML (37.5%) and CMML (7.2%). Approximately one/third (35.5%) of patients had 5q deletion, while 35.1% and 29.4% had non/5q deletion and normal karyotype, respectively. Baseline clinical characteristics of these patients are summarized in . There were a variety of AZA/plus/LEN regimens with almost all of the included studies used AZA at the dose of 75 mg/m2/day for 5–7 days [Citation10–19] but the dose of LEN varied from 5 to 50 mg per day, as detailed in .
Table 1. Baseline characteristics of studies included in the meta/analysis.
Table 2. Azacitidine-plus-lenalidomide regimens used in the included studies.
Efficacy of AZA plus LEN
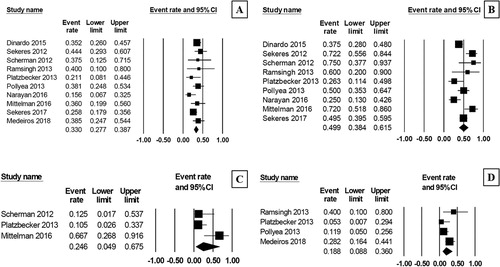
The pooled CR rate after treatment with AZA/plus/LEN regimen was 33.0% (95% CI, 27.7–38.7%, I2 = 18%; (A)) [Citation10–19], while the pooled ORR was 49.9% (95% CI, 38.4–61.5%, I2 = 72%; (B)) [Citation10–18]. The pooled analysis revealed that approximately one quarter (24.6%) of the patients achieved a complete cytogenetic response (95% CI, 49.0–67.5%, I2 = 71%; (C)) [Citation10,Citation12,Citation16]. The pooled proportion of patients showing disease progression after their treatment was 18.8% (95% CI, 8.8–36.0%, I2 = 54%; (D)) [Citation12–14,Citation19].
Toxicity of AZA plus LEN
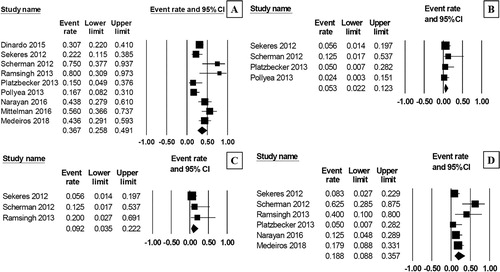
The pooled rate of grade 3–4 neutrophil toxicity and platelet toxicity were 48.8% (95% CI, 27.6–63.4%, I2 = 79%; (A)) [Citation10,Citation12,Citation13,Citation16,Citation17,Citation19] and 54.7% (95% CI, 34.1–73.9%, I2 = 76%; (B)) [Citation10,Citation12,Citation16,Citation17,Citation19], respectively. The pooled rate of febrile neutropenia was 36.7% (95% CI, 25.8–49.1%, I2 = 70%; (A)) [Citation10–17,Citation19]. The pooled rate of thrombotic events after the treatment was 5.3% (95% CI, 2.2–12.3%, I2 = 0%; (B)) [Citation10–13], while 9.2% of the patients suffered from acute renal failure (95% CI, 3.5–22.2%, I2 = 0%; (C)) [Citation10,Citation11,Citation14]. The pooled TRM rate was 18.8% (95% CI, 8.8–35.7%, I2 = 60%; (D)) [Citation10–12,Citation14,Citation17,Citation19].
Subgroup analysis
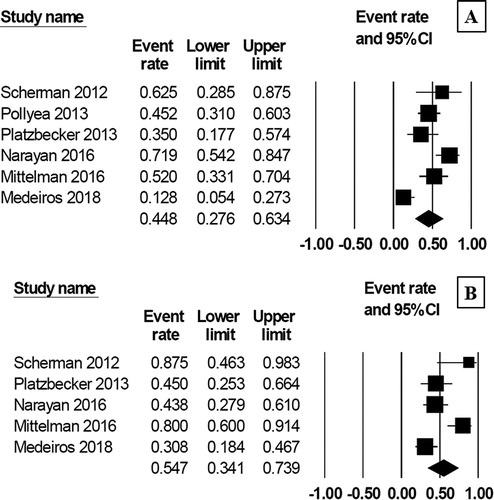
Given that approximately one/third of the patients in this study had 5q deletion and another one/third had normal karyotype, we therefore categorized the patients into two subgroups based on these cytogenetic findings and analyzed the primary outcomes, which are displayed in Supplementary Data 3. As for the patients with 5q deletion, the pooled CR rate was 43.8% (95% CI, 11.9–81.8%, I2 = 12%) [Citation10,Citation16], and the pooled ORR was 64.5% (95% CI, 46.8–79.0%, I2 = 0%) [Citation10,Citation16,Citation18]. The patients with normal karyotype had the pooled CR rate of 29.2% (95% CI, 7.4–68.1%, I2 = 46%) [Citation14,Citation16] while the pooled ORR yielded 54.7% (95% CI, 39.8–68.7%, I2 = 0%) [Citation14,Citation16,Citation18].
Discussion
This is the first systematic review and meta/analysis that comprehensively evaluated the efficacy and toxicity of the AZA/plus/LEN regimen for the treatment of AML, MDS and CMML. The pooled results across 10 studies with 406 patients found that about half of patients achieved at least partial remission and about one/third achieved CR. This observation may suggest an incremental benefit of adding LEN into AZA regimen as a recent systematic review found the ORRs of AZA monotherapy for AML, MDS and CMML in the range of low 40% [Citation25]. In addition, 25% of the included patients in the present study had a complete cytogenetic response after the treatment with AZA/plus/LEN regimen which is higher than the results of a previous study of AZA monotherapy in patients with AML and MDS patients that found the complete cytogenetic response rate in the range of 2–10% [Citation26]. Different actions of the two medications covering different stages of AML, MDS and CMML could be the foundation of the additional benefit of the combination therapy [Citation13].
On the other hand, the addition of LEN appears to increase the rate of adverse events, particularly high/grade platelet toxicity and febrile neutropenia that was seen in over half and one/third of patients, respectively. This appears to be higher than the results from a recent systematic review and meta/analysis of HMAs monotherapy the found the rate of high/grade platelet toxicity of 38% and the rate of febrile neutropenia of 25% [Citation27].
Nonetheless, comparisons of efficacy and adverse effects of the two treatment regimens from different studies are of limited validity as patients were not randomized to receive one of the treatment options. Therefore, baseline characteristics of patients between the groups were likely to be different and any observed difference in outcome/adverse effect could simply be a result of the difference in patient's characteristics, not a consequence of the therapy. Therefore, randomized, head/to/head studies to compare the efficacy of these two regimens are still needed before any definite conclusions can be drawn. In addition, the subgroup analysis in the 5q deletion group demonstrated a high CR rate and ORR. Although, the extracted number of patients was low as several included studies do not demonstrate patient's outcome separately by cytogenetic abnormalities, this promising response is of interest for further study in this group of patients to clarify the efficacy of this combination.
Conclusion
The current study may serve as preliminary data to suggest that the addition of LEN may offer incremental benefit to patients with high/risk MDS, AML and CMML. However, randomized/controlled studies that directly compare the efficacy and adverse events of AZA/plus/LEN regimen versus AZA monotherapy are still needed before any recommendations can be made.
Supplemental Material
Download Zip (105.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
The need for ethics approval by an institutional board review was waived as this study does not directly involve human subjects.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ORCID
Patompong Ungprasert http://orcid.org/0000-0002-4817-9404
Weerapat Owattanapanich http://orcid.org/0000-0002-1262-2005
Notes
1 Supplemental data for this article can be accessed https://doi.org/10.1080/16078454.2019.1631425.
References
- Society AC. Cancer Facts & Figures. 2014. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf. Accessed 1 October 2018.
- Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723
- Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish acute leukemia Registry. Clin Lymphoma Myeloma Leuk. 2011;11(Suppl 1):S54–S59. doi: 10.1016/j.clml.2011.02.003
- Owattanapanich W, Utchariyaprasit E, Tantiworawit A, et al. Improved survival of elderly-fit patients with acute myeloid leukemia requiring intensive therapy: 3-year multicenter analysis from TALWG. Clin Lymphoma Myeloma Leuk. 2018;18:e509–e514. doi: 10.1016/j.clml.2018.08.002
- National Comprehensive Cancer Network (NCCN). Acute myeloid leukemia (version 2.2018). Available from: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed 20 October 2018.
- National Comprehensive Cancer Network (NCCN). Myelodysplastic syndromes (version 2.2019). Available from: https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf. Accessed 20 October 2018.
- Kubasch AS, Platzbecker U. Beyond the edge of hypomethylating agents: novel combination strategies for older adults with advanced MDS and AML. Cancers (Basel). 2018;10. DOI: 10.3390/cancers10060158
- Ades L, Guerci-Bresler A, Cony-Makhoul P, et al. A phase II study of the efficacy and safety of an intensified schedule of azacitidine in intermediate-2 and high risk patients with myelodysplastic syndromes: a study by the Groupe Francophone des myelodysplasies (GFM). Haematologica. 2018. DOI:10.3324/haematol.2018.203885
- List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292
- Scherman E, Malak S, Perot C, et al. Interest of the association azacitidine–lenalidomide as frontline therapy in high-risk myelodysplasia or acute myeloid leukemia with complex karyotype. Leukemia. 2012;26:822–824. doi: 10.1038/leu.2011.284
- Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012;120:4945–4951. doi: 10.1182/blood-2012-06-434639
- Platzbecker U, Braulke F, Kundgen A, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013;27:1403–1407. doi: 10.1038/leu.2013.26
- Pollyea DA, Zehnder J, Coutre S, et al. Sequential azacitidine plus lenalidomide combination for elderly patients with untreated acute myeloid leukemia. Haematologica. 2013;98:591–596. doi: 10.3324/haematol.2012.076414
- Ramsingh G, Westervelt P, Cashen AF, et al. A phase 1 study of concomitant high-dose lenalidomide and 5-azacitidine induction in the treatment of AML. Leukemia. 2013;27:725–728. doi: 10.1038/leu.2012.214
- DiNardo CD, Daver N, Jabbour E, et al. Sequential azacitidine and lenalidomide in patients with high-risk myelodysplastic syndromes and acute myeloid leukaemia: a single-arm, phase 1/2 study. Lancet Haematol. 2015;2:e12–e20. doi: 10.1016/S2352-3026(14)00026-X
- Mittelman M, Filanovsky K, Ofran Y, et al. Azacitidine–lenalidomide (ViLen) combination yields a high response rate in higher risk myelodysplastic syndromes (MDS)-ViLen-01 protocol. Ann Hematol. 2016;95:1811–1818. doi: 10.1007/s00277-016-2776-x
- Narayan R, Garcia JS, Percival ME, et al. Sequential azacitidine plus lenalidomide in previously treated elderly patients with acute myeloid leukemia and higher risk myelodysplastic syndrome. Leuk Lymphoma. 2016;57:609–615. doi: 10.3109/10428194.2015.1091930
- Sekeres MA, Othus M, List AF, et al. Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American Intergroup study SWOG S1117. J Clin Oncol. 2017;35:2745–2753. doi: 10.1200/JCO.2015.66.2510
- Medeiros BC, McCaul K, Kambhampati S, et al. Randomized study of continuous high-dose lenalidomide, sequential azacitidine and lenalidomide, or azacitidine in persons 65 years and over with newly-diagnosed acute myeloid leukemia. Haematologica. 2018;103:101–106. doi: 10.3324/haematol.2017.172353
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339:b2535. doi: 10.1136/bmj.b2535
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149
- Common Terminology Criteria for Adverse Events version 3.0. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 20 October 2018.
- Mathes T, Kuss O. A comparison of methods for meta-analysis of a small number of studies with binary outcomes. Res Synth Methods. 2018;9:366–381.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–660. doi: 10.1136/bmj.327.7414.557
- Shapiro RM, Lazo-Langner A. Systematic review of azacitidine regimens in myelodysplastic syndrome and acute myeloid leukemia. BMC Hematol. 2018;18(3).
- Pleyer L, Burgstaller S, Stauder R, et al. Azacitidine front-line in 339 patients with myelodysplastic syndromes and acute myeloid leukaemia: comparison of French-American-British and World Health Organization classifications. J Hematol Oncol. 2016;9:39. doi: 10.1186/s13045-016-0263-4
- Gao C, Wang J, Li Y, et al. Incidence and risk of hematologic toxicities with hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukopenia: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11860. doi: 10.1097/MD.0000000000011860