ABSTRACT
Objective: Anemia and thrombocytopenia are the most frequently reported adverse events of ruxolitinib in patients with MPN-associated myelofibrosis (MPN-MF). Although thalidomide, androgens and prednisone have previously demonstrated improvements in myelofibrosis-associated anemia, it is unclear whether these drugs are effective in patients taking ruxolitinib.
Method: We conducted a retrospective cohort study to evaluate the efficacy and tolerability of combination therapy with low dose thalidomide, stanozolol and prednisone (TSP) in patients with IPSS intermediate-2 or high-risk myelofibrosis (MF) who received ruxolitinib treatment.
Results: Sixty-five patients with MPN-MF who took ruxolitinib were enrolled in this retrospective study, of which 46 patients also took TSP while 19 did not take TSP (TSP and non-TSP groups). Within the first 24 weeks, the proportion of patients with anemia response and platelet count increase ≥50 × 109/L were 45.7% and 67.4% in the TSP group as compared to 0% and 10.5% in the non-TSP group (p < 0.001). The mean hemoglobin level in the non-TSP group reached the nadir after approximately 12–16 weeks of therapy, but gradually increased in the TSP group.
Conclusion: In summary, TSP regimen can improve anemia and thrombocytopenia during ruxolitinib treatment in patients with MPN-MF, and the associated adverse events were manageable.
MPN-associated myelofibrosis (MPN-MF), either primary myelofibrosis or evolved from polycythemia vera (PPV-MF) or essential thrombocythemia (PET-MF), is a chronic myeloproliferative neoplasm characterized by dysregulation of the Janus kinase (JAK)/signal transducer and activation of transcription (STAT) pathway [Citation1–4]. Ruxolitinib, a potent JAK1/JAK2 inhibitor, was the first targeted drug approved for the treatment of MPN-MF based on the results of two pivotal phase III clinical trials (COMFORT-I and COMFORT-II) [Citation5,Citation6]. Ruxolitinib reduced spleen volume, improved MF-related symptoms and quality-of-life measures, and probably prolonged OS in patients with intermediate-2 or high-risk MPN-MF as compared to controls, although anemia and thrombocytopenia were the most common adverse events associated with ruxolitinib treatment. Several groups have reported that treatment of MPN-MF using thalidomide, danazol, and prednisone could improve anemia and thrombocytopenia [Citation7–11], but it is unclear whether these drugs are effective in patients taking ruxolitinib. We used ruxolitinib alone according to the medicine specification at the beginning, but some of the patients experienced severe anemia and thrombocytopenia. In an effort to reduce the hematologic adverse events after ruxolitinib treatment, we explored a treatment regimen using thalidomide, stanozolol and prednisone (TSP). We conducted a retrospective, non-randomized, comparative cohort study at our hospital for evaluating the effects of TSP in preventing anemia and thrombocytopenia in patients with MPN-MF undergoing ruxolitinib treatment.
Patients and methods
Patients were retrospectively included if they were ≥18 years of age and had PMF, PET-MF, or PPV-MF according to the 2016 World Health Organization (WHO) criteria [Citation12], with an International Prognostic Scoring system (IPSS) score of 2 (intermediate-2 risk) or ≥3 (high risk), treated with ruxolitinib combined with or without TSP regimen at our hospital. A retrospective review of patient records and continuous follow-up were performed. The Ethical Review Board of Peking Union Medical College Hospital approved the study.
The initial dose of ruxolitinib was determined by the baseline platelet count. After the first four weeks of therapy, dose regimens could be increased by 5 mg twice daily in patients who demonstrated inadequate efficacy, or reduced based on adverse events. Thalidomide 50 mg daily, stanozolol 2 mg tid, and prednisone 0.5 mg/kg/day were administered for at least three months and then discontinued if no therapeutic effect was observed. After one month of treatment, the dose of prednisone was gradually tapered to a maintenance dose of 10 mg qod in patients with ongoing treatment. The dose of thalidomide and stanozolol could be adjusted according to adverse events, such as peripheral neuropathy or liver dysfunction. TSP regimen will be continued until lose effectiveness or intolerable toxicities.
The primary endpoint of this comparative study was anemia and platelet response in patients over the first 24 weeks after ruxolitinib treatment. Anemia responses were evaluated by the IWG-MRT criteria [Citation13]. The National Cancer Institute Common Toxicity Criteria (CTCAE) was used to evaluate preventive response and other non-hematologic adverse events during the same period. The number of patients with a reduction of ≥50% in spleen length below the costal margin was also evaluated by palpation.
Statistical analysis
Cases were characterized using biochemical counts and proportions for categorical variables, and means and ranges for continuous variables. Treatment responses in both groups were compared using the chi-square test and Fisher’s exact test. P values < 0.05 were considered statistically significant. The probability of survival was estimated using the Kaplan–Meier method. Outcome differences between the groups were assessed using a log-rank test. Survival was calculated from the date of ruxolitinib treatment to patients’ death, or to the date of last follow-up evaluation for censored observations.
Results
Sixty-five patients (37 men and 28 women) with MPN-MF were treated with ruxolitinib between January 2015 and December 2018 at our hospital, of which 46 patients were also given TSP while 19 were not given TSP. Baseline variables were similar in the two groups (); except the median hemoglobin level and platelet count in the TSP group was slightly lower than in the non-TSP group, although there was no significant difference. The median age of patients was 60 (30–86) years. Fifty (76.9%) patients were positive for JAK2V617F mutation, nine (13.8%) patients had CALR mutation, six (9.2%) patients were triple-negative; 40% of the patients had IPSS intermediate-2 risk disease, and 60% had high-risk disease. Initial median values at the start of treatment were as follows: hemoglobin 96.72 g/L (50–178 g/L), platelet count 232.77 × 109/L (18–953×109/L), leukocyte count 13.48 × 109/L (0.97–60.69 × 109/L), and the median palpable spleen size was 14.18 cm below the costal margin. Transfusion dependence, defined as a red blood cell transfusion within the previous month, was noted in eight patients (12.3%).
Table 1. Baseline characteristics of the patients and doses of ruxolitinib administered.
The starting doses of ruxolitinib were similar in both groups. During the first 24 weeks, four (8.7%) patients in the TSP group and seven (36.8%) patients in the non-TSP group required a dose decrease due to toxicity(p = 0.006), and one(2.2%) patient in the TSP group and three(15.8%) patients in the non-TSP group required a discontinuation of ruxolitinib (p = 0.038). Within the first 24 weeks, according to IWG-MRT response criteria, the proportion of patients with an anemia response and platelet count increase ≥50 × 109/L were 45.7% and 67.4% in the TSP group as compared to 0% and 10.5%, respectively, in the non-TSP group (p < 0.001) (). The proportions of patients with new-onset anemia and thrombocytopenia were significantly higher in the TSP group than in the non-TSP group (). The mean hemoglobin level in the non-TSP group reached the nadir after approximately 12–16 weeks of therapy, but increased gradually in the TSP group (). Among the patients with transfusion dependence at baseline, 3/6 and 0/2 were independent of transfusions in TSP and non-TSP group respectively. Finally, the median duration of TSP maintained therapy was 65(12–289) weeks.
Figure 1. Mean change (±standard error of the mean) in (A) hemoglobin levels and (B) platelet counts from baseline over time.
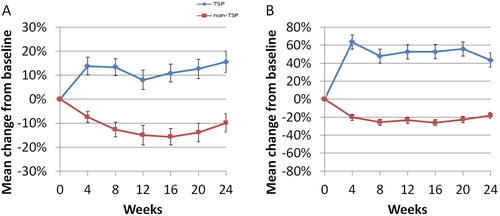
Table 2. Anemia and platelet responses in the two groups.
Table 3. Different grade hematologic adverse events of different therapeutic regimes.
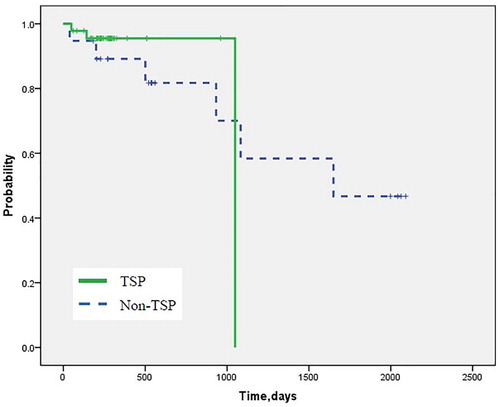
The maximum proportion of patients with a reduction of ≥50% in spleen length below the costal margin during the first 24 weeks after therapy was 68.4% (13/19) in the TSP group as compared to 58.7% (27/46) in the non-TSP group (p = 0.565). Until 24 weeks after ruxolitinib therapy, two deaths were reported in the TSP group (4.3%) as compared to one death in the non-TSP group (5.3%) (p = 0.872). Subsequently, a survival analysis of long-term follow-up revealed no significant difference between the two groups ().
Figure 2. Kaplan–Meier estimates of overall survival, including extended follow-up (p = 0.706 by log-rank test).
The common non-hematologic adverse events were increased ALT/AST and edema in 54.3% and 45.7% of patients in the TSP group as compared to 26.3% and 5.3%, respectively, in the non-TSP group (p = 0.002 and 0.039), while only three patients had ≥ grade 2 ALT/AST increase in the TSP group, and no patient discontinued therapy because of drug-related non-hematologic adverse events. Other non-hematologic adverse events were similar in the two groups ().
Table 4. Non-hematologic adverse events of different therapeutic regimes.
Discussion
Anemia is a common presentation and a negative prognostic risk factor for survival in patients with MPN-MF, and 35–54% patients present with anemia at diagnosis [Citation14,Citation15]. Ruxolitinib is the first approved JAK-STAT inhibitor that has demonstrated rapid and durable reduction in splenomegaly and improved symptoms and quality of life in both COMFORT trials. Moreover, ruxolitinib showed an overall survival benefit on long term follow up of the COMFORT studies, although the studies were not designed to test a survival advantage and ruxolitinib was not approved for this reason [Citation5,Citation16]. Consistent with the mechanism of action of ruxolitinib and the pathophysiology of MPN-MF, anemia and thrombocytopenia were the most frequently reported adverse events in all previous trials [Citation5,Citation6]. Findings from both COMFORT trials suggested that ruxolitinib-treated patients experienced an initial decrease in mean hemoglobin levels over the first 12 weeks, but the levels recovered to a new steady state by week 24 [Citation6]. Additionally, long-term follow-up in the COMFORT trial showed that the incidence of new-onset grade 3/4 anemia decreased with longer-term therapy. These data suggested that anemia is generally a short-term safety concern in patients taking ruxolitinib [Citation5].
In most clinical trials, ruxolitinib-induced hematologic adverse events could only be primarily managed by packed red blood cell transfusions or dose adjustment or even treatment discontinuation. In clinical practice, MPN-MF-associated anemia or thrombocytopenia can be managed with drugs such as thalidomide, danazol, or prednisone [Citation7–9,Citation17,Citation18]. Experience of thalidomide treatment in patients with PMF was first published in 2001 by Barosi et al., who reported improvement in anemia, thrombocytopenia, and reduction of spleen size in 43%, 67%,and 31% of patients, respectively [Citation18]. However, 91% of patients discontinued the treatment within six months mainly due to toxicity when the maximum dose of thalidomide was up to 400 mg daily. Though subsequent studies found that intermediate and high doses of thalidomide may accomplish some objectives, they were generally poorly tolerated [Citation19]. In 2003, Mesa et al. published a paper describing the effect of 50 mg thalidomide and 30 mg prednisone given daily. Increase in hemoglobin and platelet counts were observed in 70% and 75% patients, respectively, but only 19% of patients had a reduction in spleen size. However, only 5% of patients discontinued treatment due to adverse events [Citation9]. Similar results were reported in several subsequent studies, which indicated that MPN-MF patients receiving low-doses of thalidomide and prednisone achieved significant response rate in anemia with low treatment toxicity and slight reduction of spleen size [Citation8]. Moreover, Danazol are considered the treatment of choice for anemia related to MPN-MF, and several series studies have reported the use of danazol therapy in patients with MPN-MF. Luo et al. found that adding danazol could significantly increase the anemia response rate of low dose of thalidomide and prednisone in patients with MPN-MF [Citation7].
Taken together, in patients with MPN-MF, ruxolitinib can significantly reduce spleen size but induce anemia and thrombocytopenia, while low doses of thalidomide, prednisone, and Danazol achieve significant response rate in anemia and thrombocytopenia with slight reduction of spleen size. However, such ruxolitinib combination therapy has been rarely reported. To the best of our knowledge, only Gowin et al. conducted a clinical trial evaluating the efficacy of combination therapy with ruxolitinib and danazol. Although such combination therapy did not lead to increased hematologic response, hematologic stabilization was observed and may be clinically useful [Citation20]. Because of accessibility, we used stanozolol, in combination with low doses of thalidomide, prednisone and ruxolitinib to create a new regimen in patients with MPN-MF.
When ruxolitinib was used alone in MPN-MF, hemoglobin decreased immediately and reached the nadir after approximately 8–12 weeks of treatment [Citation6]. In this study, even though the baseline median hemoglobin level and platelet count in the TSP group was slightly lower than in the non-TSP group, TSP in combination with ruxolitinib not only significantly increased hemoglobin level and platelet counts according to IWG-MRT criteria but also changed the hemoglobin level trend as compared to the non-TSP group. Moreover, the new onset hemoglobin adverse events were significantly lower in the TSP group than in the non-TSP group. Consequently, although the starting doses of ruxolitinib were similar in both groups, the proportion of patients with dose reduction or discontinuation of ruxolitinib was significantly lower in the TSP group than in the non-TSP group. Although anemia and thrombocytopenia improved when ruxolitinib was given in combination with TSP, the maximum proportion of patients with a reduction of ≥50% in spleen length below the costal margin was similar in the two groups, indicating that TSP combination therapy had no obvious effect on reduction of spleen size. Moreover, the incidence of ALT/AST increase and edema was significantly higher in the TSP group than in the non-TSP group, although other non-hematologic adverse events were similar in both groups, and only few patients discontinued the combination therapy.
In summary, TSP may significantly modulate initial hematologic toxicity observed with ruxolitinib therapy. Moreover, adverse events of TSP were manageable, with minimal interruption of therapy possibly due to low dose of thalidomide. However, the small sample size and non-randomized retrospective trial design preclude definitive conclusions. Although Rampal et.al have also presented a small prospective trial utilizing thalidomide with ruxolitinib which shows the similar results [Citation21], more randomized prospective comparative clinical trials are required.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mesa RA, Green A, Barosi G, et al. MPN-associated myelofibrosis (MPN-MF). Leuk Res. 2011 Jan;35(1):12–13.
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar;365(9464):1054–1061.
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 Apr;434(7037):1144–8.
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005 Apr;352(17):1779–1790.
- Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2017 Mar;31(3):775.
- Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013 Apr;31(10):1285–1292.
- Luo X, Xu Z, Li B, et al. Thalidomide plus prednisone with or without danazol therapy in myelofibrosis: a retrospective analysis of incidence and durability of anemia response. Blood Cancer J. 2018 Jan;8(1):9.
- Belohlavkova P, Maisnar V, Voglova J, et al. Improvement of anaemia in patients with primary myelofibrosis by low-dose thalidomide and prednisone. Acta Medica Cordoba. 2016;59(2):50–53.
- Mesa RA, Steensma DP, Pardanani A, et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood. 2003 Apr;101(7):2534–2541.
- Cervantes F, Alvarez-Larran A, Domingo A, et al. Efficacy and tolerability of danazol as a treatment for the anaemia of myelofibrosis with myeloid metaplasia: long-term results in 30 patients. Br J Haematol. 2005 Jun;129(6):771–775.
- Cervantes F, Isola IM, Alvarez-Larran A, et al. Danazol therapy for the anemia of myelofibrosis: assessment of efficacy with current criteria of response and long-term results. Ann Hematol. 2015 Nov;94(11):1791–1796.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May;127(20):2391–2405.
- Tefferi A, Barosi G, Mesa RA, et al. International Working group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis Research and treatment (IWG-MRT). Blood. 2006 Sep;108(5):1497–1503.
- Reilly JT. Pathogenesis and management of idiopathic myelofibrosis. Baillieres Clin. Haematol.. 1998 Dec;11(4):751–767.
- Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005 Nov;23(33):8520–8530.
- Verstovsek S, Gotlib J, Mesa RA, et al. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J Hematol Oncol. 2017 Sep 29;10(1):156.
- Marchetti M, Barosi G, Balestri F, et al. Low-dose thalidomide ameliorates cytopenias and splenomegaly in myelofibrosis with myeloid metaplasia: a phase II trial. J Clin Oncol. 2004 Feb;22(3):424–431.
- Barosi G, Grossi A, Comotti B, et al. Safety and efficacy of thalidomide in patients with myelofibrosis with myeloid metaplasia. Br J Haematol. 2001 Jul;114(1):78–83.
- Barosi G, Elliott M, Canepa L, et al. Thalidomide in myelofibrosis with myeloid metaplasia: a pooled-analysis of individual patient data from five studies. Leuk Lymphoma. 2002 Dec;43(12):2301–2307.
- Gowin K, Kosiorek H, Dueck A, et al. Multicenter phase 2 study of combination therapy with ruxolitinib and danazol in patients with myelofibrosis. Leuk Res. 2017 Sep;60:31–35.
- Rampal RK, Verstovsek S, Devlin SM, et al. Safety and efficacy of combined ruxolitinib and thalidomide in patients with myelofibrosis: initial results of a phase II study. Blood. 2018;132(354).
