ABSTRACT
Background: Approximately, one-third of adult patients with acute myeloid leukemia (AML) are refractory to initial induction chemotherapy and relapse occurs in most patients who achieve remission. This study evaluates the efficacy of decitabine in the management of refractory or relapsed AML.
Methods: After literature search in electronic databases (Google Scholar, Embase, Ovid, and PubMed) studies were selected by following pre-determined eligibility criteria. Random-effects meta-analyses were performed to achieve effect sizes of complete remission (CR) rate, response rate (RR), and median survival after therapy. Subgroup analyses were performed with regards to use of decitabine with either epigenetics-based therapy, molecular therapy or chemotherapy.
Results: Twenty studies were included (310 patients; age 55.1 years [95% confidence interval (CI): 43.8, 66.4]; 57% [52%, 63%] males). Overall RR was 46.1% [95% CI: 36.1%, 56.1%]. Overall CR rate was 23.5% [95% CI: 22.1%, 24.9%] but was 14.85% [95% CI: 3.8%, 25.9%] for decitabine with epigenetics-based therapies, 15.4% [95% CI: 6.7%, 24.0%] for decitabine with immunotherapy or molecular therapy, 34.8% [95% CI: 18.7%, 50.9%] for decitabine with chemotherapy, and 37.5% [36.4%, 38.7%] for decitabine with chemotherapy and molecular therapy. Median survival was 7.2 months [95% CI: 5.17, 9.3]. Major adverse events were neutropenia, nausea/vomiting, infections, fatigue, febrile neutropenia, diarrhea, thrombocytopenia, anemia, anorexia, leukopenia, hemorrhage, and hyperglycemia.
Conclusion: Decitabine in combination with chemotherapy or molecular therapy has shown efficacious properties in refractory or relapsed AML patients.
Introduction
Acute myeloid leukemia (AML) is a state of proliferation with maturation arrest of clonal myeloid precursors leading to marrow failure. Such a condition if left untreated can cause death within weeks to months [Citation1]. AML can either develop spontaneously (de novo) or can arise because of cytotoxic therapy for a solid tumor (therapy-related AML) [Citation2]. AML is also associated with an antecedent hematologic disorder most frequently the myelodysplastic syndrome (MDS), and less commonly with myeloproliferative or plasma cell neoplasm [Citation3].
The median age of AML diagnosis is 69 years in the United States and the age-adjusted incidence is 3.6 per 100,000 per year. Late age is a major hurdle in considering intensive therapies for AML and therefore is associated with poor prognosis [Citation4]. In older patients (over 60 years), 3-year and 5-year survival rates are 9–10% and 3–8% respectively, whereas in younger patients, 5-year survival rates of up to 50% are reported [Citation4–6].
Cytarabine is the cornerstone of induction and consolidation for AML chemotherapy which is usually combined with an anthracycline [Citation7]. Eligibility for intensive treatment depends on age and performance status of patients. Although several regimens of chemotherapy are available for AML, efficacy is limited so far. For the treatment of AML, the interface between chemotherapy and immunotherapy is getting closer as chemotherapeutic regimens are being improved and monoclonal antibodies and gene-modified immune cells are used to address overexpression of several tumor antigens [Citation8].
Approximately, one-third of adult AML patients are resistant to initial induction chemotherapy or relapse occur in most patients who achieve remission and the majority of adult patients require salvage therapy. Even then, the chances for long-term survival are less for patients with refractory or relapsed AML. Allogeneic hematopoietic stem cell transplantation remains a possible chance for cure for these patients. Re-induction chemotherapy before a transplant in remission state is associated with significantly higher survival rates. However, patients often do not respond to re-induction therapy or to a second salvage chemotherapy regimen which leaves them unfit for additional therapy especially the stem cell transplantation [Citation9].
DNA methyltransferases (DNMT) are the enzymes which are known to cause aberrant DNA methylation leading to epigenetic silencing of normal genes which may contribute to the pathogenesis of cancer including leukemia by altering differentiation, proliferation, and apoptosis [Citation10–12]. DNA hypermethylation suppresses the expression of several tumor suppressor genes by affecting their promoter region [Citation13,Citation14]. Two azanucleoside DNMT inhibitors, azacytidine (5-azacytidine) and decitabine (5-aza-2'-deoxycytidine) have shown significant efficacy in patients with MDS [Citation15,Citation16].
Decitabine is a nucleoside analog which exhibits anticancer activity when is incorporated into DNA and forms an irreversible covalent complex with DNA (cytosine-5-)-methyltransferase 1 (DNMT1) which leads to degradation of the enzyme and consequently the hypomethylation of aberrantly hypermethylated promoters [Citation17]. Decitabine is commonly used at a dose of 20 mg/m2 for 5 days of 28 days cycle. Because of its relatively modest non-hematologic toxicity, it has shown promising outcomes of antileukemic efficacy especially for older individuals who are not candidates for more intensive treatment [Citation18]. Decitabine is also used for AML patients with refractory or relapsed disease many times in combination with other therapies. However, there is no systematic review of the studies which evaluated the outcomes of refractory or relapsed AML patients treated with decitabine. The aim of the present study was to conduct a literature survey of relevant studies to evaluate the efficacy and safety of decitabine in treating refractory or released AML patients.
Methods
This study was conducted by following the Cochrane guidelines for the conduction of systematic reviews and meta-analyses and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Inclusion and exclusion criteria
The inclusion criterion was: Clinical studies evaluating the efficacy of decitabine either alone or in combination with other therapeutic agents for the treatment of refractory or relapsed AML patients and reported efficacy and safety outcomes including the response rate and complete remission rate. A study was however excluded if reported the outcomes of in vitro investigations only; or reported the pharmaco-kinetic/-dynamic properties only; or investigated other methyltransferase inhibitors such as 5-aza-cytidine; or provided qualitative information only.
Literature search strategy
A comprehensive literature search was undertaken in multiple electronic databases including EMBASE, Google Scholar, OVID SP, PubMed and Web of Science. The major medical subject headings (MeSH) and keywords were used in different combinations. These included acute myeloid leukemia, AML, decitabine, 5-aza-2'-deoxcytidine, DAC, proliferation, maturation, response, remission, relapse, salvage, maintenance, survival, toxicity, genetic predisposition, humans, diagnosis, drug therapy, mutation, genetics, neoplastic stem cells, prognosis, and survival analysis were used in combination with primary phrase. The search encompassed original research articles published before September 2018. Reference lists of important relevant research articles and review articles were also screened.
Data extraction and statistical analysis
Data pertaining to the demographic and clinical characteristics of the study subjects, study design characteristics and outcome measuring endpoints, eligibility criteria, dosage schedules, hematological and serological marker values, analysis details and outcomes were extracted from each study report/s and tabulated in datasheets. For the assessment of the quality of the included studies, New Castle-Ottawa scale for the Assessment of Quality of Cohort Studies was used.
Endpoints of interest for the present study were: (a) response rate, (b) complete remission (CR) rate, and (c) median overall survival, after combinational use of decitabine to refractory or relapsed AML patients. To achieve overall and subgroup effect sizes of each of the endpoints, respective values and variance reported by the individual studies were pooled under random-effects model to obtain inverse variance weighted overall effect and subgroup effect sizes. Meta-analyses were performed with Stata software (version 12; Stata Corporation, Houstan, TX, U.S.A.). Between studies, inconsistency was measured with the I2 index.
Results
This systematic review has included 20 studies [Citation19–38] after screening over one thousand records and applying eligibility criteria to studies identified during the literature search (). In these studies, 310 refractory or relapsed AML patients were treated with decitabine in combination with one or more drugs. Age of these patients was 55.1 years [43.8, 66.4] and the percentage of males was 57% [52%, 63%]. Majority of these patients had relapsed AML (75% [63%, 86%]) whereas remaining had refractory disease.
Important characteristics of the included studies are presented in . Quality of the included studies was generally moderate. Comparability was the major constraint among these studies according to the New Castle-Ottawa tool of quality assessment (Table S1). Several chemotherapeutic, immunotherapeutic and molecular agents were used with decitabine to treat relapsed or refractory AML patients in these studies. Dosage schedule used in these studies is presented in .
Table 1. Characteristics of the included studies.
Table 2. Dose schedules of the included studies.
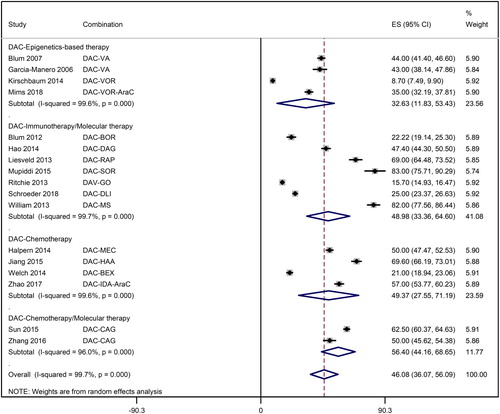
Overall response rate achieved in this meta-analysis was 46.1% [95% CI: 36.1%, 56.1%]. However, there was considerable variation not only between different forms of therapies but also within a therapeutic class. Whereas the response rate with decitabine treatment in combination with epigenetics-based therapies was 32.6% [95% CI: 11.8%, 53.4%], it was 49.0% [95% CI: 33.36%, 64.60%] for decitabine with immunotherapy or molecular therapy, 49.4% [95% CI: 27.6%, 71.2%] for decitabine with chemotherapy, and 56.40% [44.2%, 68.7%] for decitabine with chemotherapy and molecular therapy ().
Figure 2. A forest graph showing the overall and subgroup response rates achieved in the meta-analysis. Abbreviations: AMS: m-amsacrine; AraC: Cytarabine; BCNU: carmustine; BEX: bexarotene; BOR: bortezomib; BU: busulfan; CAG: cytarabine, aclarubicin, granulocyte colony stimulating factor regimen; CY: cyclophosphamide; DAC: decitabine; DAG: DLI, donor lymphocyte transplant; GO: gemtuzumab ozogamicin; HAA: harringtonine-aclarubicin-cytarabine regimen; IDA: idarubicin; MEC: mitoxantrone-etoposide-cytarabine regimen; MS: midostaurin; RAP: rapamycin; SOR: sorafenib; VA: valproic acid; VOR: vorinostat.
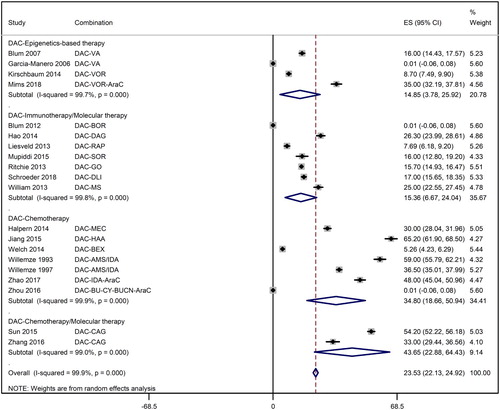
Overall complete remission rate was 23.5% [95% CI: 22.1%, 24.9%] but it ranged from 14.9% [3.8%, 25.9%] for decitabine with epigenetics-based therapies through 15.4% [95% CI: 6.7%, 24.0%] for decitabine with immunotherapy or molecular therapy, 34.8% [95% CI: 18.7%, 50.9%] for decitabine with chemotherapy, to 43.7% [95% CI: 22.9%, 64.4%] for decitabine with chemotherapy and molecular therapy ().
Figure 3. A forest graph showing the overall and subgroup complete remission rates achieved in the meta-analysis. Abbreviations as shown in .
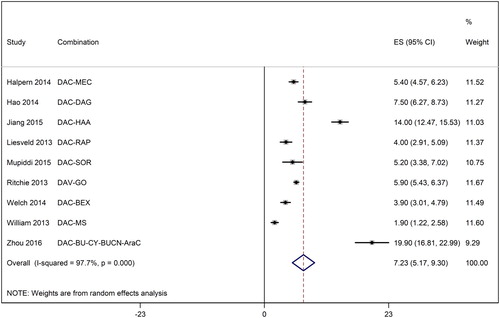
Median survival was 7.2 months [95% CI: 5.17, 9.3] ranging from 1.9 months [95% CI: 1.2, 2.6] to 19.9 months [95% CI: 16.8, 23.0] in individual studies ().
Figure 4. A forest graph showing the median survival of relapsed or refractory AML patients after decitabine-based therapies. Abbreviations as shown in .
Following adverse events were reported in the studies included in the present review (An event is followed by the number of studies in bracket reporting the incidence after combinational use of decitabine). Neutropenia (6), infection (6), fatigue (5), nausea/vomiting (5), anorexia (4), febrile neutropenia (3), diarrhea (3), pulmonary toxicity (3), gastrointestinal toxicity (3), cardiac toxicity (3), mucositis (3), thrombocytopenia (2), pneumonia (2), rash (2), renal toxicity (2), constipation (2), neurotoxicity (2), hyperglycemia (2), dyspnea (2), hemorrhage (2), hyponatremia (2), headache (2), increased aspartate aminotransferase (2), Increased alanine aminotransferase (2), hypoalbuminemia prolonged partial thromboplastin time (2), leukopenia (1), differentiation syndrome (1), encephalopathy (1), confusion (1), dizziness (1) fever (1), edema (1), hypokalemia (1), cellulitis (1), bloodstream (1), thrombosis/embolism (1), hypocalcemia (1), hypoalbuminemia (1), hyperglyceridemia (1), hypertriglyceridemia (1), asthenia (1), emesis (1), sweeting (1), hypoxia (1), hyperbilirubinemia (1), anemia (1), pain (1), colitis (1), dry mouth (1), proctitis (1), sinusitis (1), proteinuria (1), hypercreatininemia (1).
In these studies, most frequently occurring adverse events were neutropenia (61), nausea/vomiting (50), infections (45), fatigue (32), febrile neutropenia (25), diarrhea (25), thrombocytopenia (19), anemia (17), anorexia (16), leukopenia (11), hemorrhage (11), and hyperglycemia (10). Other events were less than 10 in the overall population of the present study.
Discussion
The present systematic review of the studies which reported the outcomes of a decitabine-based therapy to refractory or relapsed AML patients found that the outcomes of this treatment are associated with high statistical heterogeneity because a variety of the anti-cancer agents were used and the outcomes also varied considerably not only between different regimens but also within a class of therapy. Nevertheless, synthesis of this data revealed that decitabine in combination with chemotherapy or molecular therapy provides efficacious response rate, although the efficacious outcomes of decitabine with other epigenetics-based therapies were relatively poorer.
It is known that the addition of chemotherapeutic agent/s to decitabine improves the efficacy of this regimen by improving the benefit/risk ratio for survival in elderly AML patients [Citation39]. In vitro studies too have found that decitabine alone could not change the sensitivity or the drug-induced apoptosis, but it increased the cytotoxicity in the presence of harringtonine, aclarubicin, and cytarabine [Citation40]. Synergistic effects of decitabine and chemotherapeutic agents in improving cytotoxicity are also found in in vitro studies with AML cells [Citation41–43].
A recent meta-analysis has reported the CR rate of 27% (95% CI: 19–36), RR 37% (95% CI: 28–47) and median survival of 8.09 months (95% CI: 5.77–10.41) with decitabine monotherapy to previously untreated elderly (over 60 years of age) AML patients [Citation44]. In the present study, the overall CR rate was strongly inversely associated with age (metaregression coefficient (MC) −1.45 [−2.01, −0.89]; p<0.00001). Moreover, the response rate was also inversely associated with age but was not statistically significant (MC −0.77 [−1.86, 0.32]; p = 0.152).
These outcomes show that decitabine in combination with chemotherapeutic or molecular therapeutic agents has considerable efficacious properties for AML patients. Moreover, although, there was a trend towards increased CR rate with an increased number of combining drugs, although this association was not statistically significant (MC 7.29 [−2.05, 16.6]; p = 0.118). A number of combining drugs used in the included studies ranged from zero to four.
AML has a heterogeneous etiology and the treatment outcomes may also depend on many other factors such as age, tumor karyotype, mutational status of genes such as WT1, CEBPA, BAX, and the presence of comorbidities. Presence of some transmembrane transporter proteins which cause drug resistance by selectively extruding out drugs from the cell may also affect the prognosis. The ratio of BCL2 to BAX, BAALC, EVI1, KIT, and FLT3 genes in leukemic cells also affect the efficacy of drugs [Citation45,Citation46]. Molecular therapies for AML target several pathways such as the use of immunoconjugates like gemtuzumab ozogamicin or inhibition of enzymes such as FMS-like tyrosine kinase 3, and farnesyl transferase. Other molecular agents include multidrug resistance inhibitors, anti-angiogenesis agents, proteasome inhibitors, and apoptosis inhibitors [Citation47].
Whereas the structural changes (mutation/deletion, etc.) can permanently off gene expression, epigenetic changes are reversible and therefore gene re-expression and its associated functions can be restored with the use of pharmacologically active agents [Citation48]. Transcriptional dysregulation and epigenetic dysfunction play a role in AML etiology. This has led to the use of several pathways such as the histone deacetylase inhibition, DNA hypomethylation, B-cell lymphoma-2 inhibition, and altered DNA synthesis with the use of various nucleoside analogs [Citation49]. Overexpression of DNA methyltransferase 1 contributes to AML pathogenesis of leukemia and methylated genes involved in cellular differentiation and apoptosis are found to restore normal function upon DNA hypomethylation [Citation37].
Synergistic reversal of repression of hypermethylated genes has been observed with a hypomethylating agent treatment that often followed an HDAC inhibitor treatment, but not with concurrent use or with an HDAC inhibitor alone [Citation50]. Epigenetically repressed transcription involves complex steps mediated by several enzymatic reactions with histones such as acetylation, methylation, or ubiquitination [Citation25]. Indeed, it is suggested that mechanistically the action of epigenetic modifiers may be more complex than those of conventional cytotoxic agents [Citation51]. It is also suggested that direct cytotoxic effects may significantly contribute to decitabine efficacy which may be enhanced by other epigenetic modifiers such as vorinostat by acting on DNA damage response elements such as TP53 or GADD45A [Citation25,Citation52]. Besides inducing hypomethylation, decitabine can also increase the expression of human leukocyte antigens (HLA) class I/II antigens and increase the antigenic densities of cluster of differentiation 28/11c (CD 80/11c) antigens which promote T-cell immune recognition, proliferation, and cytokine production. Such effects can enhance the immunologic therapeutic effects of the graft-versus-leukemia effect of allogeneic hematopoietic stem cell transplantation [Citation53–55].
Conclusion
For the treatment of refractory or relapsed AML patients, decitabine is most usually used with chemotherapy or molecular therapy but the efficacy of its combinational use is much variable. However, decitabine either alone or in combination with other therapies has yielded a better response rate and complete remission rate than decitabine therapy with valproic acid or vorinostat. Future studies are required to determine the optimal combination of decitabine with other agents for the treatment of refractory or relapsed AML patients.
Supplemental Material
Download MS Word (8.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441.
- Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: The University of Chicago series. Blood. 2003;102:43–52.
- Mailankody S, Pfeiffer RM, Kristinsson SY, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS). Blood. 2011;118:4086–4092.
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113(18):4179–4187.
- Alibhai SM, Leach M, Minden MD, et al. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer. 2009;115(13):2903–2911.
- Lerch E, Espeli V, Zucca E, et al. Prognosis of acute myeloid leukemia in the general population: data from southern Switzerland. Tumori. 2009;95(3):303–310.
- Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31(Pt 2):2349–2370.
- Tettamanti S, Magnani CF, Biondi A, et al. Acute myeloid leukemia and novel biological treatments: monoclonal antibodies and cell-based gene-modified immune effectors. Immunol Lett. 2013;155(1–2):43–46.
- Feldman EJ, Gergis U. Management of refractory acute myeloid leukemia: re-induction therapy or straight to transplantation? Curr Hematol Malig Rep. 2012;7(1):74–77.
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054.
- Milosevic JD, Kralovics R. Genetic, epigenetic alterations of myeloproliferative disorders. Int J Hematol. 2013;97:183–197.
- Schoofs T, Berdel WE, Muller-Tidow C. Origins of aberrant DNA methylation in acute myeloid leukemia. Leukemia. 2014;28:1–14.
- Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2(Suppl 1):S4–S11.
- Shimizu R, Muto T, Aoyama K, et al. Possible role of intragenic DNA hypermethylation in gene silencing of the tumor suppressor gene NR4A3 in acute myeloid leukemia. Leuk Res. 2016;50:85–94.
- Atallah E, Kantarjian H, Garcia-Manero G. The role of decitabine in the treatment of myelodysplastic syndromes. Expert Opin Pharmacother. 2007;8(1):65–73.
- Gurion R, Vidal L, Gafter-Gvili A, et al. 5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome–a systematic review and meta-analysis. Haematologica. 2010;95(2):303–310.
- Hurd PJ, Whitmarsh AJ, Baldwin GS, et al. Mechanism-based inhibition of C5-cytosine DNA methyltransferases by 2-H pyrimidinone. J Mol Biol. 1999;286:389–401.
- Marks PW. Decitabine for acute myeloid leukemia. Expert Rev Anticancer Ther. 2012;12(3):299–305.
- Blum W, Klisovic RB, Hackanson B, et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25(25):3884–3891.
- Blum W, Schwind S, Tarighat SS, et al. Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood. 2012;119(25):6025–6031.
- Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108(10):3271–3279.
- Halpern AB, Othus M, Huebner EM, et al. Mitoxantrone, etoposide and cytarabine following epigenetic priming with decitabine in adults with relapsed/refractory acute myeloid leukemia or other high-grade myeloid neoplasms: a phase 1/2 study. Leukemia. 2017;31(12):2560–2567.
- Hao J, Wang L, Wang Y, et al. Comparative analysis of decitabine combined with DAG regimen and other regimens in treatment of refractory/relapsed acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2014;35(6):481–485. Chinese.
- Jiang X, Wang Z, Ding B, et al. The hypomethylating agent decitabine prior to chemotherapy improves the therapy efficacy in refractory/relapsed acute myeloid leukemia patients. Oncotarget. 2015;6(32):33612–33622.
- Kirschbaum M, Gojo I, Goldberg SL, et al. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol. 2014;167(2):185–193.
- Liesveld JL, O'Dwyer K, Walker A, et al. A phase I study of decitabine and rapamycin in relapsed/refractory AML. Leuk Res. 2013;37(12):1622–1627.
- Mims AS, Mishra A, Orwick S, et al. A novel regimen for relapsed/refractory adult acute myeloid leukemia using a KMT2A partial tandem duplication targeted therapy: results of phase 1 study NCI 8485. Haematologica. 2018;103(6):982–987.
- Muppidi MR, Portwood S, Griffiths EA, et al. Decitabine and sorafenib therapy in FLT-3 ITD-mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15 (Suppl):S73–S79.
- Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54(9):2003–2007.
- Schroeder T, Rautenberg C, Krüger W, et al. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI-a retrospective multicenter analysis on behalf of the German Cooperative transplant study Group. Ann Hematol. 2018;97(2):335–342.
- Sun Y, Xu Y, Wu D, et al. Outcomes of refractory or relapsed DNMT3A + cytogenetically normal acute myeloid leukemia patients followed the therapy including decitabine combined with CAG or CAG-like regimen. Zhonghua Xue Ye Xue Za Zhi. 2015;36(12):1025–1030. Chinese.
- Welch JS, Niu H, Uy GL, et al. A phase I dose escalation study of oral bexarotene in combination with intravenous decitabine in patients with AML. Am J Hematol. 2014;89(8):E103–E108.
- Willemze R, Archimbaud E, Muus P. Preliminary results with 5-aza-2'-deoxycytidine (DAC)-containing chemotherapy in patients with relapsed or refractory acute leukemia. The EORTC leukemia Cooperative Group. Leukemia. 1993;7(Suppl 1):49–50.
- Willemze R, Suciu S, Archimbaud E, et al. A randomized phase II study on the effects of 5-Aza-2'-deoxycytidine combined with either amsacrine or idarubicin in patients with relapsed acute leukemia: an EORTC leukemia Cooperative Group phase II study (06893). Leukemia. 1997;11(Suppl 1):S24–S27.
- Williams CB, Kambhampati S, Fiskus W, et al. Preclinical and phase I results of decitabine in combination with midostaurin (PKC412) for newly diagnosed elderly or relapsed/refractory adult patients with acute myeloid leukemia. Pharmacotherapy. 2013;33(12):1341–1352.
- Zhang LY, Yuan YQ, Zhou DM, et al. Impact of global and gene-specific DNA methylation in de novo or relapsed acute myeloid leukemia patients treated with decitabine. Asian Pac J Cancer Prev. 2016;17(1):431–437.
- Zhao H, Xu L, Yang Y, et al. Successful management of decitabine prior to Full-dose idarubicin and cytarabine in the treatment of refractory/recurrent acute myeloid leukemia. Acta Haematol. 2017;137(4):195–200.
- Zhou H, Zheng C, Zhu X, et al. Decitabine prior to salvaged unrelated cord blood transplantation for refractory or relapsed childhood acute leukemia. Pediatr Transplant. 2016;20(8):1117–1124.
- Jing Y, Zhu CY, Zhang Q, et al. Clinical efficacy of decitabine combined with modified CAG regimen for relapsed-refractory acute myeloid leukemia with AML1-ETO(+). Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:1245–1250.
- Jiang X, Wang Z, Ding B, et al. The hypomethylating agent decitabine prior to chemotherapy improves the therapy efficacy in refractory/relapsed acute myeloid leukemia patients. Oncotarget. 2015;6:33612–33622.
- Qin T, Youssef EM, Jelinek J, et al. Effect of cytarabine and decitabine in combination in human leukemic cell lines. Clin Cancer Res. 2007;13:4225–4232.
- Carter BZ, Mak PY, Mak DH, et al. Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J Natl Cancer Inst. 2014;106:djt440.
- Li K, Hu C, Mei C, et al. Sequential combination of decitabine and idarubicin synergistically enhances anti-leukemia effect followed by demethylating Wnt pathway inhibitor promoters and downregulating Wnt pathway nuclear target. J Transl Med. 2014;12(167.
- He PF, Zhou JD, Yao DM, et al. Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Oncotarget. 2017;8(25):41498–41507.
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106(4):1154–1163.
- Shipley JL, Butera JN. Acute myelogenous leukemia. Exp Hematol. 2009;37(6):649–658.
- Laubach J, Rao AV. Current and emerging strategies for the management of acute myeloid leukemia in the elderly. Oncologist. 2008;13(10):1097–1108.
- Blum W, Garzona R, Klisovica RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA. 2010;107(16):7473–7478.
- Melki JR, Clark SJ. DNA methylation changes in leukaemia. Semin Cancer Biol. 2002;12:347–357.
- Cameron EE, Bachman KE, Myohanen S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107.
- Phillips CL, Davies SM, McMasters R, et al. Low dose decitabine in very high risk relapsed or refractory acute myeloid leukaemia in children and young adults. Br J Haematol. 2013;161(3):406–410.
- Kretzner L, Scuto A, Dino PM, et al. Combining histone deacetylase inhibitor vorinostat with aurora kinase inhibitors enhances lymphoma cell killing with repression of c-Myc, hTERT, and microRNA levels. Cancer Res. 2011;71:3912–3920.
- Bhatla T, Wang J, Morrison DJ. Vepigenetic reprogramming reverse the relapse-specific gene expression signature and restores chemosensitivity in childhood B-lymphoblastic leukemia. Blood. 2012;119:5201–5210.
- Stenger EO, Turnquist HR, Mapara MY, et al. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–5103.
- Wang LX, Mei ZY, Zhou JH, et al. Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. PLoS One. 2013;8:e62924.