ABSTRACT
Background: Diabetes mellitus (DM) is considered to be a risk factor in the prognosis of many types of cancer, but the effect of DM on the risk of non-Hodgkin lymphoma (NHL) is still under dispute. We performed this study to examine the association between DM and subsequent NHL risk.
Methods: A systematically search had been performed in PubMed, EmBase, and the Cochrane Library to identify eligible studies from inception to September 2018.
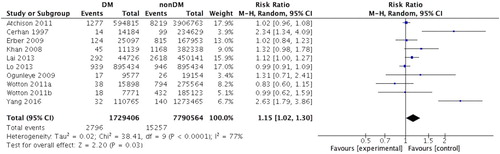
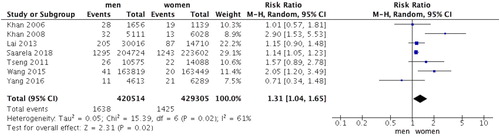
Results: Thirteen cohort studies were included, with a total of 9024761 participants. The results showed that DM was associated with an increased risk of NHL (RR = 1.15, 95%CI: 1.02, 1.30, P = .03). Subgroup analyses suggested that DM was significantly associated with patients aged less than 60 years old (RR = 1.65, 95%CI: 1.31, 2.09, P < .0001), follow-up duration within 8 years (RR = 1.23, 95%CI: 1.02, 1.48, P = .03), and studies adjusted for body mass index (RR = 1.35, 95%CI: 1.01, 1.79, P = .04). The analyses within DM patients indicated that DM men were more likely to develop NHL than DM women (RR = 1.31, 95%CI: 1.04, 1.65, P = .02).
Conclusions: These results indicated that DM patients have significantly increased risk of NHL compared nondiabetics. Male DM patients were more likely to develop NHL compared with female. However, further large-scale studies are required to eliminate miscellaneous factors in all included studies.
Non-Hodgkin lymphoma (NHL) is a common malignancy of the hematopoietic system which is derived from lymphocytes. It was more common in developed countries, accounting for 4.3% of all cancers in the U.S.A. in 2014. Among all of common cancers, NHL ranked 7th in males, while 6th in females [Citation1]. NHL has been increasing in Western countries over decades, especially in whites, men, and the elderly [Citation2]. Although decades of efforts have been made to find out the pathophysiology of NHL, the causes of NHL remained unexplained [Citation3].
Diabetes mellitus (DM) is a major global public health concern bothering approximately 3–4% of adults worldwide and contributing to around 1.3 million deaths worldwide. It is predicted to be among the five leading disease burden contributors by 2030 [Citation4]. Prevalence of DM is a risk factor for various of diseases, including cardiovascular disease, kidney failure, dementia, and cancers at different sites, such as pancreas, colorectal, liver, lung, prostate, ovarian, breast, and endometrium [Citation5–15]. Besides, according to a previous meta-analysis, DM patients had a greater potential to develop NHL [Citation16]. However, in a recent study in Japan, DM was found increased risk of NHL in males, but bot in females [Citation17]. The object of this study was to perform a large-scale of available cohort studies to estimate the relationship between DM and NHL.
Materials and methods
Literature search
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement [Citation18]. A systematic search of PubMed, Embase, and the Cochrane Library databases was performed from the first available date to 12 September 2018. For PubMed and Embase, the following key words were used: Medical Subject Heading (MeSH) or Emtree terms ‘diabetes’ or’ diabetes mellitus’ or ‘metabolic syndrome’ or ‘obesity’ or ‘hyperglycemia’ or ‘fasting glucose’ and ‘lymphoma’ or ‘non-Hodgkin lymphoma’ or ‘non-Hodgkin's lymphoma’ and ‘cohort’ combined with the corresponding free terms. Correlative keywords were used in the Cochrane Library. No language restrictions were applied to comprehensively search eligible articles.
Inclusion and exclusion criteria
Two reviewers independently selected eligible researches using the same criteria. Inclusion criteria included: (1) a cohort design; (2) assessment of NHL development with or without DM; (3) all NHLs were included, not just one, or several, subtypes; (4) reporting or providing sufficient information of odds ratios (ORs), hazard ratios (HRs), relative risks (RRs), and standard incidence ratios (SIRs); (5) patients over 18 years old. Exclusion criteria included: (1) a case report or cross-sectional design; (2) patients included have other diseases; (3) no available qualified data; (4) patients under 18 years old.
Data extraction
All data from publications were independently selected by two authors using the same criteria. The information extracted included: first author's name, publication year, country, sample size, the age and generation, body mass index (BMI), numbers of NHL, follow-up duration, the outcome measured, and the adjustment factors. We tried our best to contact authors of original studies if there were missing data in the studies.
Quality assessment
Study quality was assessed independently by two authors using the Newcastle-Ottawa Scale (NOS) [Citation19]. The NOS consists of three parameters of quality: 4 items for selection, 1 item for comparability, and 3 items for outcome. NOS scores of 1–3, 4–6, and 7–9 were assigned, respectively, for low, intermediate, and high-quality studies.
Data synthesis and analysis
Because the risk of NHL in the general population is very low, the RR was considered to approximate as OR, HR, and SIR [Citation20]. Therefore, the primary outcome was measured as RR with 95% confidence interval (CI) of the relationship between DM and NHL.
Statistical analysis
In this meta-analysis, Reviewer Manager 5.3 was applied to analyze the data. The incidence of NHL in each literature was considered as a binary variable. A fixed or random-effect model was used to pool relative risk (RR) and 95% CI for effect estimated in each study [Citation21]. Both Chi-square and I-square statistic were applied to analyze heterogeneity between studies. The heterogeneity was regarded significant when P-value < .1 [Citation22] or I2 > 50% [Citation23]. Subgroup analyses were conducted for associations of country, mean age, follow-up, and the degree of adjustment, including BMI, smoking, and drinking. Sensitivity analyses were conducted by removing each individual study from the meta-analysis. Publication bias was assessed using Stata software (version 12.0). The Egger test [Citation24] and Begg test [Citation25] were used to assess publication bias. The P-value < .05 was viewed as statistical significance.
Results
Study selection
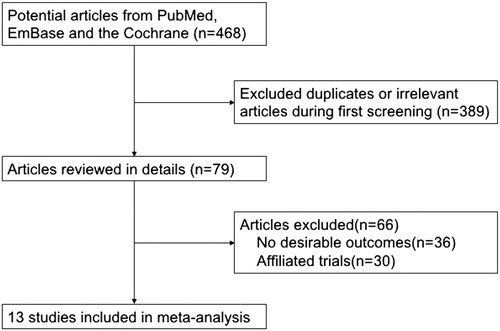
A comprehensively search was made for available eligible literatures. The process of the search was presented in . A total of 468 articles met the criteria were selected after the initial search, of which 136 duplicates were discarded, 253 articles were excluded because of insufficient or irrelevant data. A total of 79 full-text records were selected. Thirty-six articles not reporting an effect estimate, 30 studies including duplicate participants were removed. Finally, only 13 articles were included into the meta-analyses [Citation17,Citation26–37].
Study characteristics and quality assessment
Baseline characteristics were summarized in . All of included studies had a cohort design. 9,024,761 participants were involved in 4 studies performed in U.S.A., 5 in European countries, and 4 in Asian countries. The follow-up duration ranged from 1 year to 35 years. The NOS was used to assess the quality of included articles. The score of 7 or greater was considered to be highly qualified.
Table 1. Baseline characteristics of studies in the meta-analysis.
DM and NHL risk
Due to significant heterogeneity (I2 = 77%, P < .0001), a random-effect model was used to estimate pooled RR. The results showed that DM was associated with an increased risk of NHL (RR = 1.15, 95%CI: 1.02–1.30, P = .03) (). Subsequently, we performed subgroup analysis according to country, mean age, follow-up, and the degree of adjustment, including BMI, smoking, and drinking. Subgroup analysis demonstrated that DM had significant effect on NHL in studies with patients mean age less than 60 years old (RR = 1.65, 95%CI = 1.31–2.09, P < .0001), follow-up duration within 8 years (RR = 1.23, 95%CI = 1.02–1.48, P = .03), and adjusting for BMI (RR = 1.35, 95%CI = 1.01–1.79, P = .04) ().
Table 2. Subgroup analysis of relative risk for the association between DM NHL risk.
DM and NHL risk in men and women
A total of seven cohort studies assessed the incidence rate of NHL in DM patients between men and women. Pooled analysis demonstrated that significant differences existed between men and women (RR = 1.31, 95%CI = 1.04–1.65, P = .02) (). DM men were more likely to develop NHL than DM women. Though there existed moderate heterogeneity (P = .02, I2 = 61%).
DM and NHL risk in different subtypes
As shown in , only 2 articles have investigated the association between DM and NHL risk in various subtypes [Citation27,Citation28]. Since the available data are limited, more studies are needed to explore the effects of DM on different NHL subtypes.
Table 3. Relative risk for subtypes of NHL according to studies.
Publication bias
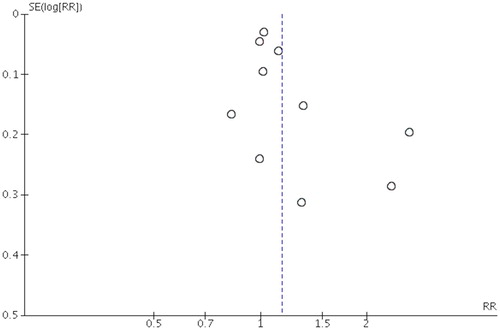
Both Egger test (P = .210) and Begg test (P = .096) showed no significant evidence of publication bias. Moreover, the funnel plots seemed symmetric (), which also indicated no publication bias.
Discussion
This meta-analysis was an update of the association between DM and NHL. The results showed that the diabetic group may have mild-to-moderate increased odds of developing NHL compared with those without diabetes, which was consistent with a previous meta-analysis [Citation16]. The subgroup analysis indicated that these association might be different according to mean age at baseline, follow-up duration, and adjustment for BMI. A former study suggested that BMI was positively related to NHL and mortality, which was consistent with the subgroup analyses [Citation38]. Furthermore, an analysis about the incidence rate of NHL was performed between diabetic men and women. The results demonstrated that diabetic men were more likely to develop NHL than women with diabetes.
Both sensitivity and subgroup analyses were used to explore the source of heterogeneity. Sensitivity analyses were performed by removing each research from the meta-analysis to see if the research might influence the heterogeneity. The results showed that no significant changes have been found when excluded each research, though moderate heterogeneity might still exist.
Previous meta-analyses combined case–control and cohort studies in order to explore the association of DM with NHL risk [Citation16]. Although the study showed similar results with ours, lack of subgroup analyses weakened its liability. Furthermore, the study failed to show the heterogeneity results, which might affect the accuracy of the conclusion. In addition, the differences between males and females were not performed.
Nowadays, a growing body of evidence indicated that DM was associated with the risk of NHL. Yang et al analyzed the association between diabetes and the risk of NHL using data from the Shanghai Men's Health Study and the Shanghai Women's Health Study, and found that DM patients have a higher risk of incident NHL after diabetes diagnosis (HR = 2.00, 95%CI = 1.32–3.03) [Citation36]. As for western countries, Erber et al. performed a large prospective study to support a moderate risk for NHL related to DM [Citation28]. However, disputation remains among several researches. A study focused on DM patients aged 30 years and over showed that DM was related to some site-specific cancers. However, the elevation of risk might be less important than complications of diabetes [Citation32]. Wang et al payed attention to the risk of multiple of cancers in mainland China, and found inadequate evidence to link NHL and DM both in men (SIR = 1.20, 95%CI = 0.88–1.62) and women (SIR = 0.95, 95% = 0.61–1.47) [Citation35]. These controversial results might be due to using the same methodology to study different cancer sites in a single population. In order to make the meta-analyses more accurate, subgroup analyses were conducted for associations of country, mean age, follow-up, and the degree of adjustment, including BMI, smoking, and drinking. And the results indicated that DM participants averaging less than 60 years old had a higher risk of developing NHL. And adjustment for BMI also had a harmful impact on NHL. However, these conclusions might be unreliable because of few eligible studies.
The present study had some strengths. A major strength was the large pooled sample size allowing a comprehensive evaluation of the association between DM and NHL. A total of 9,024,761 participants were included into the meta-analysis, which was adequate to come to a reliable result. Second, only cohort studies were included, which precluded heterogeneity from different study design. Third, several significant factors, including mean age, follow-up duration, BMI, and lifestyle, were assessed when evaluating the relationship between DM and NHL. And an evaluation was conducted to figure out different incidence rate of NHL between men and women in DM.
The present study also had certain limitations. First, the present meta-analysis did not distinguish T1DM from T2DM because few researches had done so. Due to combining the two types of DM, the relation between DM and NHL risk might be somehow influenced. However, since the most common form of DM was T2DM, particularly among older patients, it is likely that the majority of participants in this article were T2DM. Second, the miscellaneous factor could involve in the studies. Some studies adjusted age, sex, physical activity, education, lifestyle, and many other related factors, while others adjusted fewer factors. These literatures controlled some variables, however, there was still no uniform rule for exclusion factors. Third, case–control studies and individual cases were not involved in this meta-analysis, which precluded a more detailed analysis for comprehensive results. Fourthly, since the available data are limited, more studies are needed to investigate the association between DM and NHL risk in various subtypes.
Conclusion
In summary, this study suggested that DM had a potentially harmful impact on NHL risk. Physicians and health professionals should focus on DM treatment in NHL patients combined with DM, instead of merely cancer treatment.
Acknowledgements
This work was supported by “215” high-level health technology talents training plan (No.2015-3-006).
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Chihara D, Nastoupil LJ, Williams JN, et al. New insights into the epidemiology of non-Hodgkin lymphoma and implications for therapy. Expert Rev Anticancer Ther. 2015;15(5):531–544. doi: 10.1586/14737140.2015.1023712
- Chiu BCH, Weisenburger DD. An update of the epidemiology of non-Hodgkin's lymphoma. Clin Lymphoma. 2003;4(3):161–168. doi: 10.3816/CLM.2003.n.025
- Smedby KE, Askling J, Mariette X, et al. Autoimmune and inflammatory disorders and risk of malignant lymphomas – an update. J Intern Med. 2008;264(6):514–527. doi: 10.1111/j.1365-2796.2008.02029.x
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047
- Goff DC, Jr., Gerstein HC, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the action to control cardiovascular risk in diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):4i–20i. doi: 10.1016/j.amjcard.2007.03.002
- James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338
- Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307.
- Pang Y, Kartsonaki C, Guo Y, et al. Diabetes, plasma glucose and incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int J Cancer. 2017;140(8):1781–1788. doi: 10.1002/ijc.30599
- Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: a meta-analysis and systematic review. World J Gastroenterol. 2015;21(19):6026–6031. doi: 10.3748/wjg.v21.i19.6026
- Zhang ZJ, Zheng ZJ, Shi R, et al. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(7):2347–2353. doi: 10.1210/jc.2012-1267
- Lee JY, Jeon I, Lee JM, et al. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer. 2013;49(10):2411–2423. doi: 10.1016/j.ejca.2013.02.025
- Gang P J, Mo L, Lu Y, et al. Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocr Res. 2015;40(1):54–61. doi: 10.3109/07435800.2014.934961
- Lee JY, Jeon I, Kim JW, et al. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2013 Mar;23(3):402–412. doi: 10.1097/IGC.0b013e31828189b2
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi: 10.1002/ijc.22717
- Friberg E, Orsini N, Mantzoros CS, et al. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50(7):1365–1374. doi: 10.1007/s00125-007-0681-5
- Mitri J, Castillo J, Pittas AG. Diabetes and risk of non-Hodgkin's lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31(12):2391–2397. doi: 10.2337/dc08-1034
- Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan collaborative cohort (JACC) study. Asian Pac J Cancer Prev. 2006;7(2):253–259.
- Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001
- Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002
- Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. doi: 10.1177/0272989X05282643
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997 Nov 1;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446
- Cerhan JR, Wallace RB, Folsom AR, et al. Medical history risk factors for non-Hodgkin's lymphoma in older women. J Natl Cancer Inst. 1997;89(4):314–318. doi: 10.1093/jnci/89.4.314
- Khan AE, Gallo V, Linseisen J, et al. Diabetes and the risk of non-Hodgkin's lymphoma and multiple myeloma in the European prospective investigation into cancer and nutrition. Haematologica. 2008;93(6):842–850. doi: 10.3324/haematol.12297
- Erber E, Lim U, Maskarinec G, et al. Common immune-related risk factors and incident non-hodgkin lymphoma: the multiethnic cohort. Int J Cancer. 2009;125(6):1440–1445. doi: 10.1002/ijc.24456
- Ogunleye AA, Ogston SA, Morris AD, et al. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer. 2009;101(7):1199–1201. doi: 10.1038/sj.bjc.6605240
- Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128(3):635–643. doi: 10.1002/ijc.25362
- Tseng CH. Diabetes, insulin use, and non-Hodgkin lymphoma mortality in Taiwan. Metab Clin Exp. 2012;61(7):1003–1009. doi: 10.1016/j.metabol.2011.11.015
- Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia. 2011;54(3):527–534. doi: 10.1007/s00125-010-1987-2
- Lai GY, Park Y, Hartge P, et al. The association between self-reported diabetes and cancer incidence in the NIH-AARP diet and health study. J Clin Endocrinol Metab. 2013;98(3):E497–E502. doi: 10.1210/jc.2012-3335
- Lo SF, Chang SN, Muo CH, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. Int J Cancer. 2013;132(1):182–188. doi: 10.1002/ijc.27597
- Wang M, Hu RY, Wu HB, et al. Cancer risk among patients with type 2 diabetes mellitus: a population-based prospective study in China. Sci Rep. 2015;5:11503. doi: 10.1038/srep11503
- Yang WS, Li HL, Xu HL, et al. Type 2 diabetes and the risk of non-Hodgkin's lymphoma: a report from two population-based cohort studies in China. Eur J Cancer Prev. 2016;25(2):149–154. doi: 10.1097/CEJ.0000000000000150
- Saarela K, Tuomilehto J, Sund R, et al. Cancer incidence among Finnish people with type 2 diabetes during 1989–2014. Eur J Epidemiol. 2018;34(3):259–265. doi: 10.1007/s10654-018-0438-0
- Larsson SC, Wolk A. Body mass index and risk of non-Hodgkin's and Hodgkin's lymphoma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47(16):2422–2430. doi: 10.1016/j.ejca.2011.06.029