ABSTRACT
Objective: Immunoglobulin D (IgD) levels are often elevated in patients with autoimmune diseases. However, the oncogenic activities of IgD and IgD receptor (IgDR) in diffuse large B-cell lymphoma (DLBCL) have not been reported in detail. Therefore, we aimed to investigate the expression of IgD and IgDR in patients with DLBCL.
Methods: Membrane IgD (mIgD) and IgDR expression in tissue samples was analyzed using IHC, mIgD and IgDR expression on peripheral blood mononuclear cells (PBMCs) was analyzed by FCM, and secreted IgD (sIgD) level was analyzed by ELISA. Fisher’s exact test and Spearman correlation analysis were used to evaluate the relationship between IgD, IgDR, and clinical parameters.
Results: The pathological lymph nodes of 34 patients with DLBCL were studied, and mIgD and IgDR expression was found in 16 and 19 patients. mIgD and IgDR expression was upregulated in patients with DLBCL and mIgD expression was significantly associated with IgDR expression. Further correlation analysis showed that mIgD expression was correlated with serum β2-MG level and Hans algorithm as germinal center B (GCB), whereas IgDR expression correlated with serum LDH level, IPI score and GCB. ELISA showed that sIgD level was significantly increased in DLBCL patients and it correlated with serum β2-MG and LDH levels. FCM showed that mIgD and IgDR expression in PBMCs of patients with DLBCL was significantly higher than that in healthy controls.
Conclusion: Our findings suggest that overexpression of IgD and IgDR is an abnormal activation state in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most commonly occurring subtype (30–40%) of non-Hodgkin’s lymphoma (NHL), which is an aggressive lymphoma with immunephenotypic, genetic features and heterogeneous clinicopathological [Citation1]. The molecular and cellular intricacies underlying DLBCL development and progression have been widely studied. Many selected novel agents based on the association of the active site of B-cell with R-CHOP (rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone) were tested for DLBCL treatment and were found to have high safety and efficacy [Citation2], but all of them had side effects such as allergic indigestion, leukopenia, arrhythmia and systemic reactions and some patients could not tolerate the toxicity of immunochemotherapy. Therefore, it is more important to explore more novel effective therapeutic target and highly selective drugs for further DLBCL treatment.
Immunoglobulin D (IgD), a novel immunoglobulin (Ig) can be expressed as membrane IgD (mIgD) or secreted IgD (sIgD). mIgD is an important B-cell marker, as it is co-expressed with IgM on most of mature B-cells [Citation3]. Dysregulated expression of IgD has been detected in the biopsies specimens of patients with some malignant lymphomas [Citation4]. Furthermore, mIgD expression and IgD-mediated calcium signaling was found to be amplified in chronic lymphocytic leukemia [Citation5] and sIgD was found to be increased in IgD myeloma and Hodgkin’s disease [Citation6,Citation7]. Anti-IgD antibody can induce apoptosis in B-cell lymphomas and in chronic lymphocytic leukemia [Citation8,Citation9]. Like other Ig (IgA, IgE, IgG, and IgM), IgD also has a specific Fc receptor (IgDR), which is present on a small percentage (<5%) of T-cells [Citation10,Citation11]. It can bind sIgD and mIgD to their specific antigen and mediate T-cell and B-cell activation and interaction [Citation12]. IgDR is also present on neoplastic B-cells and may act with other membrane receptors in triggering responses [Citation13]. However, the functional and molecular characteristics of IgD and IgDR still remain elusive.
Because the gene encoding the IgDR has not yet been cloned, the structural and functional characteristics of the IgDR are still unclear. The expression of IgDR has been described on CD4+ and CD8+ T cells in mice and humans, and also found in the human basophilic cell [Citation14–16]. Binding of IgD to IgDR, expressed on human CD4+ T cells, was also determined using IgD as the ligand. Binding between IgD and IgDR was clearly concentration-dependent. We have estimated the IgD binding affinity by performing a fluorescence-based receptor binding assay, with high-affinity (KD value was 0.216 nmol/L) [Citation17]. We also identified IgD-binding IgDR as a ∼70 kDa protein complex that was detected on rheumatoid arthritis (RA)-fibroblast-like synoviocytes (FLSs) by using flow cytometry (FCM), confocal laser scanning microscopy and western blot [Citation18]. Our findings provided further evidence for the existence of IgDR. IgDR expressed on T, B cells and FLSs contribute to FLS activation [Citation18]. Previous reports also suggested significantly high levels of sIgD, mIgD, and IgDR in RA patients. In human, treatment with IgD could enhance the activation of peripheral blood mononuclear cells (PBMCs) [Citation15], and amplify the activation of CD4+ T cells, which could be mediated by Lck phosphorylation [Citation17]. In Daudi cell line, which is the human Burkitt lymphoma B-cell line, we have investigated that human IgD antibody could induce cell proliferation potentially by activating the putative IgDR to initiate tyrosine phosphorylation signaling cascade that accelerated G1/S transition [Citation19].
Taken together, these findings about IgD and IgDR on B-cell tumor cell lines suggest that this ligand–receptor crosslinking may be involved in the pathogenesis of B-cell related diseases. Accordingly, the aim of the present study was to evaluate the clinical presence as well as the possible alterations of IgD, and IgDR in DLBCL patients, and the association between mIgD or IgDR expression and clinical parameters of DLBCL.
Materials and methods
Patients and samples
Paraffin-embedded archived samples from 34 cases of DLBCL prior to therapeutic interventions were obtained from the Affiliated Provincial Hospital of Anhui Medical University. All these patients were diagnosed according to the WHO criteria, between June 2014 and December 2014 [Citation20]. Meanwhile, lymph node tissue samples were collected from 14 patients with reactive lymphoid hyperplasia which matched the test samples in gender and age. Clinical information, including patient gender, age, Ann arbor stage, B symptoms, site, immunotype, serum levels of lactic dehydrogenase (LDH) and β2-microglobulin (β2-MG), and International Lymphoma Prognostic Index (IPI) scores, was also obtained.
Blood samples from 16 patients with DLBCL were taken during the initial diagnosis, whereas serum from 10 healthy volunteers was collected to be used as normal controls. All these patients were diagnosed between March 2015 and September 2015. The experimental protocol used in this study was approved by the Ethics Committee of Anhui Medical University, and written informed consent was obtained from all the participants involved in this study.
Antibodies and reagents
Human IgD full-length protein (hIgD, ab 91022) was purchased from Abcam (Cambridge, MA, USA). Biotinylated IgD was prepared in our laboratory using a protein biotinylation kit from Pierce Biotechnology (Rockford, IL, USA), according to the manufacturer’s instructions [Citation21]. Streptavidin APC-Cy7, APC-anti-IgD antibody, and IgG1 isotype control were purchased from BD Pharmingen (San Diego, CA, USA).
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tissue sections of 4 μm thickness were deparaffinized and hydrated. High-pressure antigen retrieval was performed using a citrate buffer (pH 6.0). Endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol for 20 min, followed by incubation with a normal serum to block non-specific staining.
For assessment of mIgD, primary antibodies used was mouse anti-human monoclonal antibodies against IgD (dilution 1:100). The sections were incubated with the primary antibody overnight in a humidified chamber at 4°C. The tissue sections of mIgD were washed and treated with reagent A and B from the SP reagent kit (Zhongshan Goldenbridge Biotechnology Company, China).
Expression of IgDR was detected by treating tissue sections with biotinylated IgD [Citation21]. For detecting IgD binding receptors, primary antibodies used was mouse anti-human monoclonal antibodies against biotinylated hIgD (dilution 1:100). The sections were incubated with the primary antibody overnight in a humidified chamber at 4°C. The tissue sections of mIgD were washed and treated with horseradish peroxidase-conjugated streptavidin. Then, the sections were stained using a diaminobenzidine kit (DAB; Zhongshan Goldenbridge Biotechnology Company, China), counterstained with hematoxylin, and mounted. Immunohistochemical staining in a series of randomly selected 5 high-power fields, considered to be representative of the average staining in tumors at ×400 magnification, were analyzed by two independent observers who were blinded to all the clinical data. The sections were analyzed using an Image-pro Plus Image analysis and management system (Media Cybernetics Company, USA) and were scored according to the proportion of positively stained tumor cells. According to the CD20 positive standard [Citation22], cases were considered as positive when more than 20% of the tumor cells were stained. All cases were assigned to the Germinal Center B-cell like (GCB) or non-GCB group as proposed by Hans et al. [Citation23].
Isolation of PBMCs
PBMCs were isolated from whole blood using Ficoll gradient centrifugation. Blood was diluted 1:1 with phosphate-buffered saline (PBS) and layered onto Hypaque at a ratio of 4:3 (blood + PBS: Hypaque) and centrifuged at 800g for 20 min at 25°C. The PBMC layer was removed carefully, washed three times, and resuspended in 1.5 ml PBS and 1% BSA (1 × 106 cells/100 μl). The cell viability was above 95%, as evaluated by the Trypan blue dye exclusion method.
FCM analysis
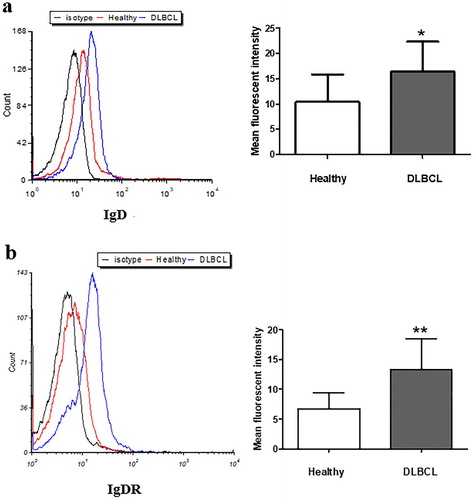
PBMCs collected from healthy controls and patients with DLBCL were washed twice and suspended in PBS at a concentration of 1 × 106/ml. For assessment of mIgD, cells were resuspended in PBS and stained using APC anti-IgD at 37°C for 30 min after blocking the nonspecific binding sites. For IgDR direct staining, cells were incubated with biotinylated IgD for 1 h on ice [Citation19]. Then, the cells were washed three times with PBS and incubated with streptavidin APC-Cy7 for 30 min on ice. Cells stained with streptavidin APC-Cy7 alone were used as an isotype control. All the cells were washed twice with cold PBS and were resuspended in PBS for FCM analysis performed using the flow cytometer (model FC 500; Beckman Coulter Ltd., Brea, CA, USA) and FlowJo software (version 7.6.1; Tree Star, Ashland, OR, USA).
ELISA for sIgD
Serum samples from 10 healthy volunteers and 16 patients with DLBCL were collected and frozen at −80°C. sIgD levels were quantified using a human ELISA kit (Abcam, MA, USA), according to the manufacturer’s instructions.
Statistical analysis
All statistical analyses were performed using SPSS, version 17.0 for Windows. The numerical data were statistically analyzed by the 2-tailed Student’s t-test and presented as mean ± S.D. Associations between IgD and IgDR expression and clinical or biological variables were assessed by Chi-square analysis, and the Fisher’s exact test and Spearman correlation analysis were used, where required. Differences with P < 0.05 were considered statistically significant.
Results
Migd and IgDR expression in DLBCL tissues
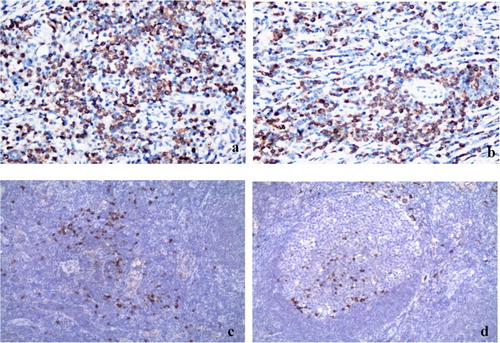
Immunohistochemical analysis revealed that the mIgD and IgDR proteins are located on the membrane and cytolymph of tumor cells within DLBCL tissues (). At the time of diagnosis, expression of mIgD and IgDR were observed in 16 patients and 19 patients respectively of 34 cases of DLBCL patients. However, their positive expression in 14 patients with reactive hyperplasia were both 2 cases. Fisher’s exact test revealed significant differences between mIgD, IgDR and reactive hyperplasia groups, suggesting mIgD and IgDR coexpression.
Figure 1. The expression of mIgD and IgDR from DLBCL tissues and reactive hyperplasia of lymph nodes. Tissue samples were analyzed using IHC and a Image-pro Plus Image analysis and management system (×100). a. The expression of mIgD from DLBCL tissues. b. The expression of IgDR from DLBCL tissues. c. The expression of mIgD from reactive hyperplasia of lymph nodes. d. The expression of IgDR from reactive hyperplasia of lymph nodes.
Association between mIgD or IgDR expression and clinical parameters
Following immunohistochemical analysis, the association between mIgD or IgDR expression and clinical parameters was analyzed. As shown in , there were no differences in age, sex, Ann arbor stage, B symptoms and site between groups. However, mIgD expression was observed more frequently in patients with a higher serum β2-MG level compared with the negative mIgD expression in patients with a lower serum β2-MG level. Moreover, the association between IgDR expression and serum LDH level was statistically significant. The IPI score, which is the most important prognostic indicator, was strongly associated with IgDR expression. Both mIgD and IgDR expression are observed more frequently in Hans algorithm as germinal center B (GCB) patients than non-GCB patients.
Table 1. Correlation between mIgD and IgDR expression with clinical pathological parameters of DLBCL patients.
Migd and IgDR expression in PBMCs
To investigate mIgD and IgDR expression of PBMCs, we isolated and compared PBMCs from 10 healthy volunteers and 16 patients with DLBCL. The FCM results showed that expression of both mIgD and IgDR was considerably higher in PBMCs from patients with DLBCL was than those from healthy controls ().
Serum levels of sIgD in DLBCL
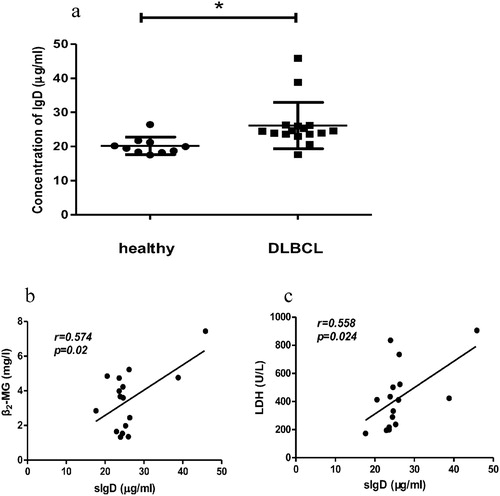
We measured and compared the serum levels of sIgD in patients with DLBCL and healthy controls ((a)). The ELISA result showed that the sIgD level of DLBCL patients was significantly higher than that of healthy controls.
Association between serum sIgD level and clinical parameters
To investigate the possible involvement of sIgD in DLBCL, we investigated the association between serum sIgD levels of patients with DLBCL and some important clinical parameters such as serum β2-MG level, serum LDH level, and IPI score. The Fisher’s exact test and Spearman correlation analysis results showed a positive correlation between serum sIgD level and serum β2-MG and LDH levels in patients with DLBCL ((b,c)). However, there was no correlation between serum sIgD levels and IPI score in these patients.
Discussion
The biological processes that lead to lymphomagenesis are complex, and it is universally acknowledged that immune system dysfunction is one of the most important events in NHL pathogenesis. Although IgD accounts for approximately 1% of the normal serum immunoglobulins, it is involved in different immunological mechanisms [Citation14,Citation16,Citation24] and may be involved in NHL occurrence [Citation25]. In this study, we investigated IgD and IgDR expression in DLBCL and its association with different clinical parameters representative of DLBCL.
IgD promotes immune defense, which causes inflammation and tissue damage by inducing the activation and infiltration of immune cells [Citation16,Citation26]. As a B-cell receptor (BCR) expressed on the surface of B cell, mIgD plays an important biological role in lymphocyte differentiation [Citation27], peripheral lymphocyte activation [Citation28], and modulation of the immune response [Citation26]. Previously, strong mIgD immunoreactivity was reported in mantle cell lymphoma [Citation29]. Immunohistochemical studies reported the expression of mIgD in splenic marginal-zone lymphoma and nodal DLBCLs [Citation30]. The mIgD expression in lymph node biopsies is associated with pediatric nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) [Citation31]. Our previous reports also have demonstrated that there is an elevated IgD level in RA patients [Citation15]. Nevertheless, the expression IgD has not yet been investigated in DLBCL.
The earliest research about the presence of IgDR on T-cells was in 1985 [Citation32], and it indicated that IgD also serves as a ligand for IgD-specific receptors. Because the gene encoding the IgDR has not yet been cloned, the structural and functional characteristics of the IgDR are still unclear. Previously, we used biotinylated IgD and FCM analysis to detect IgDR expression [Citation33] and first reported IgDR expression on RA-FLSs, T cells and B cells [Citation18,Citation15,Citation19]. IgDR is also be found to present on neoplastic B-cells [Citation13]. However, the oncogenic activities of IgD and IgDR in DLBCL have not been reported in detail. The immunohistochemical and FCM analysis performed during the present study showed that the expression of mIgD and IgDR in DLBCL patients is upregulated and that mIgD expression is significantly associated with IgDR expression.
Furthermore, high sIgD levels were reported in Hodgkin’s lymphoma [Citation7] and chronic rhinosinusitis [Citation34]. Recently, we found that serum sIgD levels in patients with RA were significantly higher than that in healthy controls [Citation15]. In this study, ELISA showed significantly high serum sIgD levels in patients with DLBCL. On the basis of these results, we speculate that such an abnormal level of IgD and IgDR in DLBCL may contribute to DLBCL progression.
Well-recognized prognostic factors for DLBCL include age, performance status, LDH, Ann Arbor stages, and a number of affected extranodal sites, which are incorporated into IPI, the most popular system used to predict prognosis [Citation35]. Other prognostic factors include gender, serum β2-MG, tumor bulk, and B symptoms. Therefore, to know the clinical significance of mIgD and IgDR in DLBCL, we assessed the association between mIgD and IgDR expression and clinical parameters. This correlation analysis revealed a positive correlation between mIgD expression and serum β2-MG levels, as well as between IgDR expression and serum LDH levels and IPI score. Serum IgD level correlated with serum β2-MG and LDH levels. Many published studies have demonstrated that serum β2-MG and LDH levels are associated with the prognosis of DLBCL [Citation36,Citation37] and IPI is a well-established prognostication system for risk stratification of DLBCL [Citation38]. We also found mIgD and IgDR expression was more common in the GCB type of Hans classification. Thus, our correlation analysis provides clinical evidence for the possible involvement of IgD and IgDR in lymphomagenesis. The limitation of this study is that the sample size was small, more patient samples may help to illustrate the pathology mechanism of IgD and IgDR in DLBCL. The correlation between IgD/IgDR expression and disease classification, progress and curative effect should be analyzed based on sufficient cases. Furthermore, it is interesting as the correlation of the nodal and blood levels of these proteins in the same patient. Additionally, the IgDR related signaling pathway may help to find the molecular mechanisms in DLBCL disease progressing by using animal models.
In conclusion, our study shows overexpression of IgD and IgDR in patients with DLBCL and suggests that detection of IgD and IgDR was correlated with DLBCL disease severity. Serum level changes of IgD might prove useful for monitoring the disease course and treatment outcome.
Acknowledgments
The authors acknowledge Doctor Da-Bin Huang and Doctor Jie Chen in Affiliated Provincial Hospital of Anhui Medical University, for their help in pathological specimens and blood samples collecting.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sun F, Zhu J, Lu S, et al. An inflammation-based cumulative prognostic score system in patients with diffuse large B cell lymphoma in rituximab era. BMC Cancer. 2018;18(1):5.
- Chiappella A, Santambrogio E, Castellino A, et al. Integrating novel drugs to chemoimmunotherapy in diffuse large B-cell lymphoma. Expert Rev Hematol. 2017;10(8):697–705.
- Kerr WG, Hendershot LM, Burrows PD. Regulation of IgM and IgD expression in human B-lineage cells. J Immunol. 1991;146(10):3314–3321.
- Untanu RV, Back J, Appel B, et al. Variant histology, IgD and CD30 expression in low-risk pediatric nodular lymphocyte predominant Hodgkin lymphoma: a report from the Children’s Oncology group. Pediatr Blood Cancer. 2018;65(1):e26753.
- Haerzschel A, Catusse J, Hutterer E, et al. BCR and chemokine responses upon anti-IgM and anti-IgD stimulation in chronic lymphocytic leukaemia. Ann Hematol. 2016;95(12):1979–1988.
- Ishii K, Yamato K, Kubonishi I, et al. IgD myeloma presenting as a testicular tumor: establishment and characterization of an IgD-secreting myeloma cell line. Am J Hematol. 1992;41(3):218–224.
- Gobbi PG, Merlini G, Lattanzio G, et al. Serum IgD in Hodgkin’s disease. Acta Haematol. 1981;66(1):35–38.
- Carey GB, Scott DW. Role of phosphatidylinositol 3-kinase in anti-IgM- and anti-IgD-induced apoptosis in B cell lymphomas. J Immunol. 2001;166(3):1618–1626.
- Tavolaro S, Peragine N, Chiaretti S, et al. Igd cross-linking induces gene expression profiling changes and enhances apoptosis in chronic lymphocytic leukemia cells. Leuk Res. 2013;37(4):455–462.
- Lakshmi TSM, Wu Y, Toporovsky I, et al. Igd receptor-mediated signal transduction in T cells. Cell Immunol. 2001;207(2):110–117.
- Coico RF, Tamma SL, Bessler M, et al. IgD-receptor-positive human T lymphocytes. I. Modulation of receptor expression by oligomeric IgD and lymphokines. J Immunol. 1990;145(11):3556–3561.
- Coico RF, Siskind GW, Thorbecke GJ. Role of IgD and T delta cells in the regulation of the humoral immune response. Immunol Rev. 1988;105:45–68.
- Rudders RA, Andersen J. IgD-Fc receptors on normal and neoplastic human B lymphocytes. Clin Exp Immunol. 1982;50(3):579–586.
- Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol Rev. 2010;237(1):160–179.
- Wu Y, Chen W, Chen H, et al. The elevated secreted immunoglobulin D enhanced the activation of peripheral blood mononuclear cells in rheumatoid arthritis. PLoS One. 2016;11(1):e0147788.
- Chen K, Xu W, Wilson M, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10(8):889–898.
- Wu YJ, Chen HS, Chen WS, et al. CP-25 attenuates the activation of CD4+T cells stimulated with immunoglobulin D in human. Front Pharmacol. 2018;9:4.
- Wu YJ, Chen WS, Chen HS, et al. The immunoglobulin D Fc receptor expressed on fibroblast-like synoviocytes from patients with rheumatoid arthritis contributes to the cell activation. Acta Pharmacol Sin. 2017;38(11):1466–1474.
- Dai X, Wu Y, Jia X, et al. hIgD promotes human Burkitt lymphoma Daudi cell proliferation by accelerated G1/S transition via IgD receptor activity. Immunol Res. 2016;64(4):978–987.
- Koch K, Klapper W. Classification of malignant lymphomas. Internist (Berl). 2016;57(3):206–213.
- Lisanti MP, Sargiacomo M. Biotinylation and analysis of membrane-bound and soluble proteins. Curr Protoc Immunol. 2001; Chapter 8: Unit 8.16.
- Choi CH, Park YH, Lim JH, et al. Prognostic implication of semi-quantitative immunohistochemical assessment of CD20 expression in diffuse large B-cell lymphoma. J Pathol Transl Med. 2016;50(2):96–103.
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282.
- Rigante D. The truth on IgD in the ploy of immune surveillance and inflammation. Immunol Res. 2016;64(2):632–635.
- Papadaki T, Stamatopoulos K, Mavrommatis T, et al. A unique case of IgD-only splenic marginal-zone lymphoma with mutated immunoglobulin genes: ontogenetic implications. Leuk Res. 2008;32(1):155–157.
- Nguyen TG, Little CB, Yenson VM, et al. Anti-IgD antibody attenuates collagen-induced arthritis by selectively depleting mature B-cells and promoting immune tolerance. J Autoimmun. 2010;35(1):86–97.
- Goding JW. Allotypes of IgM and IgD receptors in the mouse: a probe for lymphocyte differentiation. Contemp Top Immunobiol. 1978;8:203–243.
- Jelinek DF, Splawski JB, Lipsky PE. Human peripheral blood B lymphocyte subpopulations: functional and phenotypic analysis of surface IgD positive and negative subsets. J Immunol. 1986;136(1):83–92.
- Zheng Y, Zhou X, Xie J, et al. Igm expression in paraffin sections distinguishes follicular lymphoma from reactive follicular hyperplasia. Int J Clin Exp Pathol. 2014;7(6):3264–3271.
- Zettl A, Meister S, Katzenberger T, et al. Immunohistochemical analysis of B-cell lymphoma using tissue microarrays identifies particular phenotypic profiles of B-cell lymphomas. Histopathology. 2003;43(3):209–219.
- Papadaki T, Stamatopoulos K, Belessi C, et al. Splenic marginal-zone lymphoma: one or more entities? A histologic, immunohistochemical, and molecular study of 42 cases. Am J Surg Pathol. 2007;31(3):438–446.
- Coico RF, Xue B, Wallace D, et al. T cells with receptors for IgD. Nature. 1985;316(6030):744–746.
- Wu Y, Lakshmi TSM, Lima V, et al. Facilitated antigen presentation by B cells expressing IgD when responding T cells express IgD-receptors. Cell Immunol. 1999;192(2):194–202.
- Sokoya M, Ramakrishnan VR, Frank DN, et al. Expression of immunoglobulin D is increased in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2017;119(4):317–323.e1.
- Abadi U, Peled L, Gurion R, et al. Prevalence and clinical significance of hypercalcemia at diagnosis in diffuse large B-cell lymphoma. Leuk Lymphoma. 2019: 1–5.
- Chen W, Luo RC, Fan WW, et al. Clinical value of combined detection of LDH, TPS, CEA and beta2-MG in patients with non-Hodgkin’s lymphoma. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26(2):227–228. 230.
- Park JH, Yoon DH, Kim DY, et al. The highest prognostic impact of LDH among international prognostic indices (IPIs): an explorative study of five IPI factors among patients with DLBCL in the era of rituximab. Ann Hematol. 2014;93(10):1755–1764.
- Biccler J, Eloranta S, de Nully Brown P, et al. Simplicity at the cost of predictive accuracy in diffuse large B-cell lymphoma: a critical assessment of the R-IPI, IPI, and NCCN-IPI. Cancer Med. 2018;7(1):114–122.