ABSTRACT
Objective: To investigate clinical features, diagnosis, treatment strategies and prognosis of juvenile myelomonocytic leukemia (JMML).
Methods: The clinical data of 21 patients with JMML who were diagnosed in our hospital from January 2013 to May 2018 were retrospectively analyzed.
Results: Among the 21 children with JMML, 16 were male and 5 were female. Out of the 21 children who were diagnosed with JMML, 7 were lost after treatment while the remaining 14 received A-3V chemotherapy regimen of South Korea. The effective response rate was 78.5%. The three-year overall survival (OS) rate and three-year disease-free survival (DFS) rate were (76.2 ± 14.8)% and (66.2 ± 14)%, respectively. Single factor analysis showed that PLT count ≤33×109/L, LDH level >500 U/L and HbF level >10% and chemotherapy only were the significant factors that lead to poor prognosis in children. Cox multivariate analysis showed that the choice of treatment options affected the prognosis of JMML children. By taking prognostic factors for long-term efficacy into account, patients with treatment strategy of chemotherapy alongside hematopoietic stem cell transplantation (HSCT) have a better prognosis.
Conclusion: The PLT count, LDH level, HbF level and choice of treatment plan are important for the evaluation of prognosis for children with JMML. Although there is a lack of consistency in terms of donors but the A-3V scheme is relatively stable, so HSCT should be preferred for children with poor prognostic factors.
Juvenile myelomonocytic leukemia (JMML) is a pluripotent hematopoietic stem cell that can cause erythroid hyperplasia, abnormal platelet count, abnormal lymphocyte function, myelodysplastic syndrome (MDS) and myeloproliferation. It fits all the characteristics of myeloproliferative neoplasms (MPN). JMML was first reported in 1953 as a chronic myelogenous leukemia in children. It was then uniformly named as juvenile chronic granulocyte monocytic leukemia in 1994. In 2008, WHO included the disease in the MDS/MPN category. The incidence of JMML is low at only 1.2/106 with a median age of onset at 2 years and 6 months old, occurring more often in male than female [Citation1]. The clinical manifestations are fever, hepatosplenomegaly and rash. It is relatively difficult to diagnose and treat as compared to childhood acute leukemia, hence leading to many cases of misdiagnosis and delayed treatment. We retrospectively analyzed the case data of 21 children with JMML who were newly diagnosed in our hospital from January 2013 to May 2018. Clinical characteristics, molecular genetic characteristics, treatment strategies and prognostic factors of these cases were included in this study to help clinicians in improving the accuracy level of diagnosis and treatment of the disease.
1. Materials and methods
Participants enrolled in our hospital from January 2013 to May 2018. There were a total of 21 patients with JMML, including 16 males and 5 females. The median age of onset was 2 years and 9 months old (11 months old to 10 years old), and the median time from onset to definite diagnosis was 2 months (10 days to 8 months).
All cases of JMML were diagnosed according to the diagnostic criteria [Citation2] developed by the international JMML assistance group ().
Table 1. Single-factor analysis of prognosis factors affecting long-term efficacy of JMML children.
The chromosome karyotype of all children were analyzed using the G-banding method. High-throughput sequencing was also done using the Illumina miseq platform to analyze the six mutant genes associated with JMML: KRas, NRas, NF1, PTPN11, SETBP1 and CBL.
Treatment regimen involves chemotherapy and induced differentiation therapy that were performed using the Korean A-3 V regimen [Citation3]. The standard protocol includes cytarabine (Ara-C) 100 mg / (m2.d) (d0 ∼ 6), etoposide (VP-16) 100 mg / (m2. d) (d0 ∼ 4), vincristine (VCR) 1.5 mg / (m2. d) (d9), 13-cis retinoic acid 75 ∼ 100 mg / (m2. d) (d10 ∼ 20).
Efficacy evaluation and follow-up procedures are based on the consensus by the international JMML collaboration group in 2015 [Citation4]. The parameters of early treatment response evaluation include 6 clinical features and 3 genetic characteristics. It is divided into three levels according to the efficacy: complete remission, partial remission and disease progression. Complete remission is defined by effective early treatment. The follow-up period was as of 31 May 2018.
Statistical analysis was performed using SPSS 22.0 software. Clinical characteristics were analyzed and compared using descriptive analysis, corrected chi-square test and exact probability method; survival analysis was done by using Kaplan–Meier survival curve whereas single factor analysis was done through the Log-rank test. Factors with P < 0.05 in the univariate analysis were included in the multivariate analysis and the Cox regression model was used. P < 0.05 was considered as statistically significant.
2. Result
During the initial diagnosis, it was observed in 21 children cases that JMML exhibited clinical manifestations of being pale and hepatosplenomegaly, whereby there were 12 cases of ≥ 3 cm under the rib margin and 8 cases of ≥3 cm under the spleen margin. Besides, there were also 15 cases of fever, 2 cases of milk coffee spots, 2 cases of maculopapular rash, 12 cases of skin and mucous membrane bleeding, and 4 cases of rash, trunk and dispersed distribution. 4 cases out of the total of 21 were misdiagnosed as immune thrombocytopenic purpura and one other case as systemic lupus erythematosus.
2.2. Hematology examination
The median white blood cell count was 27.6 (3.01∼94.6)×109/L, median hemoglobin level was 93 (47∼111) g/L, and median platelet count was 60(3∼207)×109/L, the absolute value of the median monocyte count was 3.5 (1∼9.4)×109/L. There were 14 cases that have tested positive for C-reactive protein (CRP) and 7 cases turned out negative.
In all 21 of the JMML patients, median Lactic Acid Dehydrogenase (LDH) level was 517 (140 ∼ 1000) U/L. HbF levels in 18 cases were higher than their normal values with a median value of 31.2% (3%∼78.1%); only HbF levels in 3 cases are normal.
12 of the patients tested positive for Epstein–Barr virus (EBV), accounting for 57.1% of the patient population while 2 patients tested positive for cytomegalovirus (CMV), accounting for 9.5% of the patient population. There was 1 case of EBV-CMV combined positive, accounting for 4.7% of the patient population.
Peripheral blood cell morphology in 16 children showed naive cells with a median naive cell count of 9% (2% to 18%). Among the remaining 5 cases, 1 was normal, 2 cases had seen neutrophils leaving the nucleus in the presence of toxic particles and 2 other cases had shown an increase in the proportion of monocytes despite exhibiting normal morphology. Among the 21 patients that had undergone red blood cell morphology examination, 13 cases exhibited different sizes, central light staining and heterotypic (+); there was 1 case of polychromatic erythrocyte while the remaining 7 cases were normal.
Bone marrow cell morphology and flow cytometry showed 9 cases with active bone marrow, 7 cases of bone marrow hyperplasia and 5 cases of hyperosteogeny. The median value of granulocyte + promyelocytic cell count was 8% (1% to 19%) while the median value of primary monocyte + naive monocyte count was 10% (5% to 17.4%). The megakaryocyte level was reduced in 15 cases, active in 3 cases and normal in 3 cases. There were 12 cases of erythroid hyperplasia and 9 cases of hyperplasia. Out of the 21 children with JMML, megaloblastic changes were exhibited in 7 cases, multinucleated red blood cells were detected in 1 case, lymphoid small megakaryocytes were found in 1 case, Pelger-Huet malformation was observed in 2 cases and hemophagocytic cells ocassionally appeared in 1 case. The results of bone marrow flow cytometry showed that the median value of myeloid primitive cell count was 12.5% (2.3%∼18.9%).
2.4. Genetic examination
There were no abnormalities in the bone marrow karyotype for 14 cases while chromosome abnormalities were observed in the remaining 7. 3 cases were chromosome 3 (+8 chromosome), 1 case was accompanied by chromosome 7 deletion (-7 chromosome); 1 case was 46, XY, t(3; 5)(q25; q34); For example, 45, X, -Y, t (8; 21) (q22; q22); 1 case is 46, XX, t (16; 21) (p11; q22); 1 case is 46, XY, t (8;21) (q22; q22).
Abnormal JMML-related mutations were detected in 16 patients (76.2%), 10 of which were double-gene mutations, 5 were single-gene mutations and 1 was multi-gene mutations. There were 5 cases that involve the mutations of NF1 and PTPN11 gene, 4 cases involving the mutations of NRas and NF1 gene, 1 case involving the mutations of KRas and NF1 gene, 1 case of NRas single gene mutation, 3 cases of NF1 single gene mutation, 1 case of CBL single gene mutation and 1 case of NF1 and CBL polygene mutations. The frequency of NF1 mutation was the highest among all mutant genes, accounting for 93.3% (14/15 cases) of the patient population, followed by NRas at 37.5% (6/16 cases), PTPN11 at 31.25% (5/16 cases), KRas at 6.25% (1/16) and CBL at 12.5% (2/16 cases).
2.5. Treatment effect
2.5.1. Early treatment response and its influencing factors
Out of the 21 children who were diagnosed with JMML, 7 were lost after treatment while the remaining 14 received chemotherapy from A-3 V in South Korea. Among them, 11 patients achieved complete remission after 1–2 courses of treatment, 3 patients had partial remission and 1 patient had no disease progression. The effective response rate of early treatment was 78.5% (11/14 cases). We performed a univariate analysis of factors that may influence early treatment response such as gender, age, liver and spleen size, white blood cell count, hemoglobin, platelet count, LDH, HbF, karyotype and JMML mutant genes. Results showed that age is the most significant factor that affects early treatment response (X2 = 5.091, P = 0.024) as early treatment response was better in patients aged ≤2 years old.
2.5.2. Long-term survival and influencing factors
2.5.2.1. Long-term survival
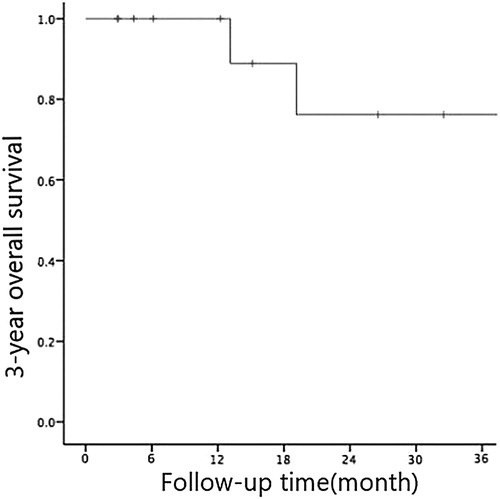
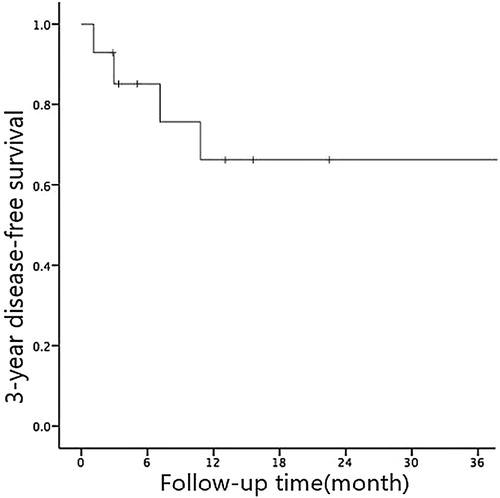
The follow-up period was up until 31 May 2018. The median OS time of 14 children with JMML was 42.9 (3∼54) months while the median DFS was 37.9 (2∼54) months. The 3-year OS and DFS rates were (76.2 ± 14.8)% and (66.2 ± 14)% respectively (see and ). 9 out of the 14 patients had undergone chemotherapy only while the other 5 patients underwent both chemotherapy and HSCT. Five patients were dead during the follow-up period due to recurrence. Four of them had bone marrow recurrence and one had bone marrow + central recurrence.
2.5.2.2. Univariate analysis of long-term effects on children with JMML
We ran a univariate analysis of factors that may affect long-term outcomes in children with JMML such as gender, age, liver and spleen size, white blood cell count, hemoglobin level, platelet count, LDH level, HbF level, karyotype and JMML mutant gene. PLT count ≤33×109/L, LDH level > 500 U/L, HbF level >10% and simple chemotherapy are significant factors that lead to poor prognosis in children (see ).
2.5.2.3. Multivariate analysis of long-term effects of children with JMML
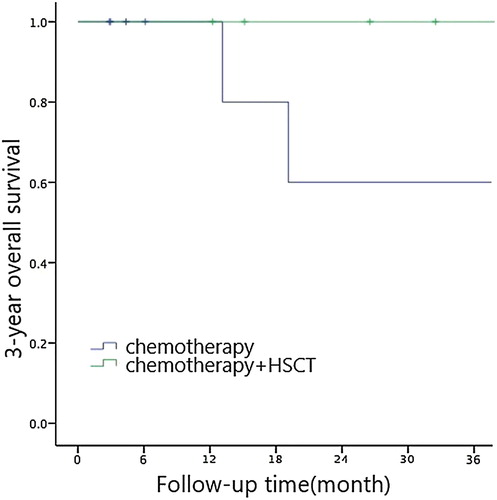
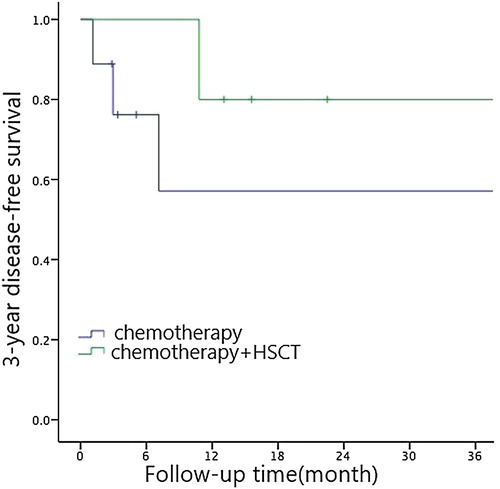
Cox multivariate analysis showed that the choice of treatment regimen (RR = 1.087, 95% CI: 0.244-4.832, P = 0.044) was an independent prognostic factor affecting long-term outcomes in children. Chemotherapy + HSCT had shown to be leading to better prognosis (see , and ).
Table 2. Conditioning regimen and outcome of the 5 patients with JMML.
3. Discussion
JMML is a kind of invasive myeloid disease in children. It is characterized by the hyperproliferation of monocyte system, infiltration of important organs and a series of other clinical symptoms. The atypical symptoms and signs of JMML often causes misdiagnosis or missed diagnosis. In this study, 21 patients with JMML were diagnosed. Of the 17 patients who were transferred to our hospital due to unclear diagnosis, 4 patients were misdiagnosed as other diseases in the other hospitals. Analysis of thesechildren showed that early clinical manifestations were diversed hence leading the initial clinical diagnosis to either be immune thrombocytopenic purpura, systemic lupus erythematosus or viral infection.
There were 12 cases of EBV infection in our cohort 2 cases of EBV and CMV coinfection. The incidence of viral infection was 57.1%, consistent with the report of Manabe et al. [Citation5]. The pathogenesis of viral infection in JMML was considered by the study. Infection with EBV or other viruses causes disease by stimulating pre-existing malignant clones. Further analysis of the onset characteristics of the children found that most of the skin bleeding points (12 cases) or rash (4 cases) for the prominent performance of the treatment, have hepatosplenomegaly, blood test more common thrombocytopenia (18 cases) Significantly increased mononuclear cells (21 cases), less than 20% of peripheral blood immature cells, suggesting that we should be alert to the possibility of JMML in the case of such patients. If the bone marrow naive cells are <20% and the BCR-ABL gene is negative, HbF level and JMML related mutation genes should be tested in time for early diagnosis.
It is currently believed that genetic testing can improve the accuracy of JMML diagnosis. In this group of patients, peripheral blood smears did not show myeloid primordial cells during the onset of the disease and HbF level was within the normal range for their age, causing difficulties during the initial diagnosis. Subsequent genetic testing found NF1, CBL and NRas mutations which finally leads to the diagnosis of JMML. The incidence of different gene mutations varies. Previous studies [Citation6,Citation7] indicated that 35% of children with JMML had PTPN11 mutations, 30% had KRas/NRas mutations, 10% to 15% had NF1 mutations and 10% had CBL mutations. Our results show that NF1 has the highest frequency at 93.3%, followed by NRas, PTPN11, CBL and finally KRas. However, due to the small case number included in this study, it cannot be concluded that NF1 and NRas are the most prominent JMML-related gene mutations in the Chinese population. The number of cases needs to be expanded. From our data, there is no significant correlation between the type of gene mutation and clinical manifestation severity and treatment prognosis, but studies have shown that PTPN11 mutation is a clear prognostic factor [Citation8].
Children with JMML have a poor response to most standard chemotherapy regimens and hematopoietic stem cell transplantation is currently the only effective treatment available. However, Kang [Citation3] and others had reported a new treatment method by combining chemotherapy and induced differentiation (A-3 V program). Although only 5 cases were included in the study, this program is relatively safe and effective in the absence of matching donors. We applied this regimen in 14 children with JMML. The results showed that the bone marrow activity was mildly inhibited and there were no signs of serious infection. Among them, 11 patients achieved complete remission after 1–2 courses while the remaining 3 patients had partial remission. No disease progression was observed and the effective response rate of early treatment was 78.5%. Follow-up on 9 patients who underwent the regimen alone showed that 7 patients continued to remission for more than 1 year, including 1 patient who had completed 12 courses and continued to remission for 32 months, 4 patients who had completed 6 courses and continued to remission for 16 months, and 2 patients who had completed 7 courses and continued to remission for 20 months. This suggests that the A-3 V program can help in early remission of children with JMML. It can also be used as an induction therapy before transplantation. For those who lack a suitable donor and whose conditions are stable, the A-3 V regimen is worthy of clinical recommendation. Our univariate analysis showed that age was the most significant factor affecting early treatment response. Patients at the age of ≤ 2 years old had a good early response. Studies [Citation9,Citation10] suggested that this may be due to the presence of DNA methylation in tumor cells of children with JMML as hypermethylation mainly occurs in older children.
The literature reports that the prognosis of children with JMML is quite different. About 1/3 of children with JMML have a rapid onset of disease and may have a poor prognosis [Citation11–13]. We have done a single factor analysis of the long-term effects of children with JMML and found out that PLT count ≤33 × 109/L, LDH level >500 U/L, HbF level >10% and chemotherapy only contribute to poor prognosis in children. The choice of treatment regimen are independent risk factors for prognosis. Due to the proliferation of tumor cells in children with JMML, hypoxia-ischemia damage occurs in tissues surrounding the body cells, cell permeability increases, and cellular aerobic metabolism is converted into anaerobic glycolysis. Anaerobic leaven the solution inducing a large amount of LDH to be released by the cells. This made the LDH level an important indicator for evaluating the prognosis of JMML. Decreased platelet count (PLT count ≤ 33 × 109/L) and increased levels of HbF (HbF level >10%) [Citation9,Citation10] are associated with hypermethylation which suggests a poor prognosis. It was observed in children with JMML that the protein kinase A-type kinase-anchored protein 12 that works downstream of the RAS signaling pathway, showed different degrees of hypermethylation and CREBBP gene. So far, hematopoietic stem cell transplantation is still the only way to cure JMML. Some studies [Citation14] reported that the overall survival rate of JMML in hematopoietic stem cell transplantation in recent years has reached 50% to 64%, while the transplant-related mortality rate was 11% to 13%. The 3-year OS rate of children with JMML in our hospital receiving hematopoietic stem cell transplantation is 100% and the 3-year EFS is 80 ± 17.9%, higher than the above-mentioned literature reports. The reason may be that we are progressing rapidly and we made JMML patients who were reported to have poor prognosis (initial diagnosis at the age of >2 years old, PLT count ≤33×109/L, HbF level >10%, chromosome -7, PTPN11 mutations and combined gene mutations) undergo the Korean A-3 V chemotherapy program before HSCT was actively selected. Immunosuppressant was withdrawn as soon as possible within 3 months after transplantation, which helped prevent recurrence and improved efficacy. Therefore, we believe that for patients with the stable condition and no acute changes, the Korean A-3 V chemotherapy program contributes to sustained remission, is well tolerated, and has a low mortality rate. Children can wait for the opportunity to receive hematopoietic stem cell transplantation. As for children with JMML who are critically ill with poor prognosis, appropriate donors should be sought as soon as possible, and hematopoietic stem cell transplantation is preferred.
Availability of data and materials
The data sets used and/or analyzed during the current study are not available because of privacy.
Acknowledgements
The study has been approved by Sun Yat-sen Memorial Hospital Ethics Committee (China). Authors gratefully acknowledge the help of all parents and children.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Kun-yin Qiu http://orcid.org/0000-0002-2865-4685
Additional information
Funding
References
- Yan X, editor-in-chief. Pediatric hematology. Tianjin: Science and Technology Press; 2005. p. 466–468.
- Chang TY, Dvorak CC, Loh ML. Bedside to bench in juvenile myelomonocytic leukemia: insights into leukemogenesis from a rare pediatric leukemia. Blood. 2014;124(16):2487–2497. doi: 10.1182/blood-2014-03-300319
- Kang HJ, Shin HY, Choi HS, et al. Novel regimen for the treatment of juvenile myelomonocytic leukemia (JMML). Leuk Res. 2004;28(2):167–170. doi: 10.1016/S0145-2126(03)00217-0
- Niemeyer CM, Loh ML, Cseh A, et al. Criteria for evaluating response and outcome in clinical trials for children with juvenile myelomonocytic leukemia. Haematologica. 2015;100(1):17–22. doi: 10.3324/haematol.2014.109892
- Manabe A, Yoshimasu T, Ebihara Y, et al. Viral infections in juvenile myelomonocytic leukemia: prevalence and clinical implications. J Pediatr Hematol Oncol. 2004;26(10):636–641. doi: 10.1097/01.mph.0000140653.50344.5c
- Braun BS, Shannon K. Targeting Ras in myeloid leukemias. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(8):2249–2252. doi: 10.1158/1078-0432.CCR-07-1005
- Loh ML, Sakai DS, Flotho C, et al. Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood. 2009;114(9):1859–1863. doi: 10.1182/blood-2009-01-198416
- Park HD, Lee SH, Sung KW, et al. Gene mutations in the Ras pathway and the prognostic implication in Korean patients with juvenile myelomonocytic leukemia. Ann Hematol. 2012;91(4):511–517. doi: 10.1007/s00277-011-1326-9
- Wilhelm T, Lipka DB, Witte T, et al. Epigenetic silencing of AKAP12 in juvenile myelomonocytic leukemia. Epigenetics. 2016;11(2):110–119. doi: 10.1080/15592294.2016.1145327
- Fluhr S, Boerries M, Busch H, et al. CREBBP is a target of epigenetic, but not genetic, modification in juvenile myelomonocytic leukemia. Clin Epigenetics. 2016;8(1):50. doi: 10.1186/s13148-016-0216-3
- Sakashita K, Matsuda K, Koike K. Diagnosis and treatment of juvenile myelomonocytic leukemia. Pediatr Int Off J Japan Pediatr Soc. 2016;58(8):681–690. doi: 10.1111/ped.13068
- Matsuda K, Shimada A, Yoshida N, et al. Spontaneous improvement of hematologic abnormalities in patients having juvenile myelomonocytic leukemia with specific RAS mutations. Blood. 2007;109(12):5477–5480. doi: 10.1182/blood-2006-09-046649
- Niemeyer CM, Kang MW, Shin DH, et al. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42(9):794–800. doi: 10.1038/ng.641
- Locatelli F, Nöllke P, Zecca M, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105(1):410–419. doi: 10.1182/blood-2004-05-1944