ABSTRACT
Objectives: Congenital factor XIII (FXIII) deficiency is a rare severe bleeding disorder. Intracranial hemorrhage (ICH) is the leading cause of mortality and morbidity in FXIII deficiency. However, its pathogenesis is not well understood yet. In this study, we investigated the expression and CpG island methylation status of matrix metalloproteinase-2 (MMP-2) and MMP-9 in patients with FXIII deficiency and ICH.
Methods: Forty patients with FXIII deficiency including twenty patients with ICH, and twenty without ICH were recruited as case and control groups, respectively. Methylation status was determined by bisulfite sequencing polymerase chain reaction (PCR), and gene expression was assessed by quantitative real-time PCR.
Results and discussion: We found an unmethylated pattern for both MMP-2 and MMP-9 genes in the case group. Both genes were partially methylated in the control group, while the percentage of methylated CpGs was significantly higher in MMP-9 than MMP-2 (P = 0.001). Furthermore, higher expression of MMP-9 (in both the mRNA and protein levels) was found in the case than control group (P = 0.008 and P = 0.009, respectively). On the other hand, there was no significant difference in MMP-2 expression level (neither mRNA nor protein) between the two groups (P = 0.12 and P = 0.25, respectively).
Conclusion: Our findings indicated that MMP-9 over-expression might be related to ICH in FXIII deficiency, and gene methylation effectively regulates its expression. Future researches will expand our understanding of the pathogenesis of ICH in congenital FXIII deficiency.
Introduction
Coagulation factor XIII (FXIII) is a tetrameric pro-transglutaminase that consists of two catalytic A (FXIII-A2) and two protective/carrier B (FXIII-B2) subunits. It is activated in the final phase of the coagulation cascade and cross-links fibrin chains, resulting in a stronger clot. FXIII is a multifunctional protein with pleiotropic roles in mediating a wide variety of physiological and pathological processes including the wound healing, angiogenesis, embryo implantation, neoplasm, and cerebrovascular diseases [Citation1,Citation2].
Congenital FXIII deficiency is a rare bleeding disorder (RBD) with an autosomal recessive pattern of inheritance and estimated incidence of 1 per 2 million in the general population. The disorder is more common in Iran, especially Khash city (Khash factor XIII) with an incidence of 1 homozygote per ∼500 individuals and 3.5% heterozygotes, in this area [Citation3–7]. Based on residual plasma FXIII level in patients with FXIII deficiency, bleeding symptoms can range from mild to severe such as intracranial hemorrhage (ICH). ICH is the main cause of death and >80% of deaths in congenital FXIII deficiency are attributed to ICH and umbilical cord bleeding. Furthermore, ICH is more common in congenital FXIII deficiency than any other congenital bleeding disorder. Occurrence of ICH in congenital FXIII deficiency can be affected by several factors, including residual plasma factor level, age of patient and prophylaxis regimen [Citation3–8].
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases were originally described as matrix-degrading enzymes. These highly destructive enzymes are tightly controlled at different levels, including transcription, translation, activation, and inhibition. Available evidences indicate that dysregulation of their potential proteolytic activity might contribute to neurological disorders such as stroke and ICH through impairment of the neurovasculature [Citation9–12].
MMP-2 (gelatinase A) and MMP-9 (gelatinase B), are shown to be associated with disruption of blood brain barrier (BBB) and subsequently ICH. Interestingly, MMP-2 and MMP-9 are able to digest collagen. Therefore, there is a great interest in the role of MMPs in ICH induction [Citation13–16]. Some studies have suggested that promoter methylation is a regulatory mechanism for MMP expression [Citation17–20].
In this context, we aimed to assess the relationship between MMP-2 and MMP-9 gene expression or promoter methylation status and ICH in congenital FXIII deficiency.
Materials and methods
Study patients
Forty patients with congenital FXIII deficiency including 20 patients with ICH and 20 without ICH were recruited, as case and control groups, respectively. Diagnosis of ICH was done based on general clinical presentations of ICH and neuroimaging, as described previously (2). Clinical manifestations of the patients were assessed by an expert staff as well as a physician by physical examination and a structural questionnaire, at time of disease diagnosis, and six-month regular intervals. The mean age of case and control groups was 12.6 ± 0.8 and 10.2 ± 1.2 years, respectively. The mean age at time of ICH in case group was 12.6 ± 0.8. Patients with amyloid angiopathy, liver diseases, brain tumors, head trauma, and heavy smokers were excluded from the study. Informed consent was obtained from all participants and the study was approved by the local ethics committee of Tehran University of Medical Sciences (ethics code: IR.TUMS.REC.1394.638).
Bisulfite treatment
Venous blood samples were collected in EDTA-containing tubes, within 24–48 h after ICH (15). Genomic DNA was isolated from leukocytes using the QIAamp DNA Mini kit (QIAGEN, Hilden, Germany) and then, treated with sodium bisulfite using the EpiTect Bisulfite Kit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol.
Bisulfite sequencing PCR (BSP)
Finding of the CpG islands, sparse CG dinucleotides, and the BSP specific primers design were performed using MethPrimer (www.urogene.org/methprimer/index1.html) and UCSC (www.genome.ucsc.edu) online tools. Characteristics of target CpG islands are detailed in .
Table 1. Characteristics of target CpG islands.
Target CpG islands in MMP-2 and MMP-9 gene promoters were amplified using the specific primers:
MMP-2 (F: 5′-GGATTATGAGTTGTTGAGTC-3′ and R: 5′-ACTCACCACTACCAACTC-3′) and MMP-9: (R: 5′-TTAGGTGTGGGTGTATATAG-3′ and R: 5′-AATCCTCCTCAACCCTCA-3′).
Then, PCR products were sequencing (ABI 3730xl; Applied Biosystems). Chromas, CodonCode Aligner and Lalign softwares were used for sequence alignment and finding the TG or CG dinucleotides.
RNA extraction and cDNA synthesis
Total RNA was extracted from peripheral blood using RNeasy® Plus Mini Kit (QIAGEN, Hilden, Germany). The quality and quantity of extracted RNA were determined by the gel electrophoresis and NanoDrop (Nano Drop Technologies, Thermo Scientific, Wilmington, DE, USA), respectively. Then, cDNA synthesis was performed by QuantiTect Reverse Transcription Kit (QIAGEN, Hilden, Germany).
Quantitative real-time RT–PCR
Specific primers were designed using Primer Express software as follows: MMP-2 (F: 5′-ATTCTGGAGATACAATGAGGTGAAG-3′; R: 5′-GCACCCTTGAAGAAGTAGCTG-3′) and MMP-9 (F: 5′-CAAGGGCGTCGTGGTTCC-3′; R: 5′-CCGTCCTGGGTGTAGAGTC-3′). Quantitative real-time-PCR was performed by LineGene K (BIOER) real-time-PCR system using 2X QuantiFast SYBR Green PCR Master Mix (QIAGEN, Hilden, Germany).
The reaction condition was initial denaturation (94°C for 10 min) followed by 40 cycles of 5 s at 95°C, 40 s at 72°C and then a final extension step of 10 min at 72°C. GAPDH was used as an internal control (F: 5′-CGGAGTCAACGGATTTGGTCGTT-3′, R: 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′). Relative expression of MMP-2 and MMP-9 was defined as fold change, normalized to GAPDH expression levels.
Determination of MMP-2 and MMP-9 plasma concentrations
Commercially available enzyme-linked immunosorbent assay (ELISA) kits (abcam, Cambridge, UK) were used to quantify the plasma concentration of MMP-2 (ab100606) and MMP-9 (ab100610). All measurements were performed in triplicate.
Statistical analysis
Data analysis was performed by SPSS software. Comparison between case and control groups was performed using chi-square and independent t-tests. P-value of 0.05 or less was considered to be statistically significant.
Results
Patients’ characteristics
All study population had severe congenital FXIII deficiency. There were no statistically significant differences between median age and the gender of case and control groups (P = 0.06, P = 0.4 respectively). Main demographic characteristics of the study groups are summarized in .
Table 2. Demographic distribution between two groups.
BSP products for MM-2 and MMP-9 genes
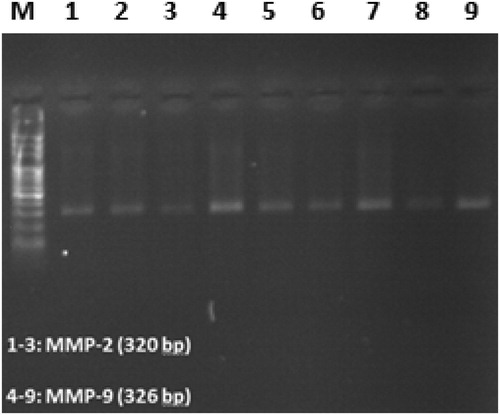
Amplified regions of MMP-2 and MMP-9 promoters CpG islands in BSP showed 320 and 326 bp bands in Agarose gel electrophoresis, respectively ().
Methylation status of MMP-2 and MMP-9 genes in patients with congenital FXIII deficiency
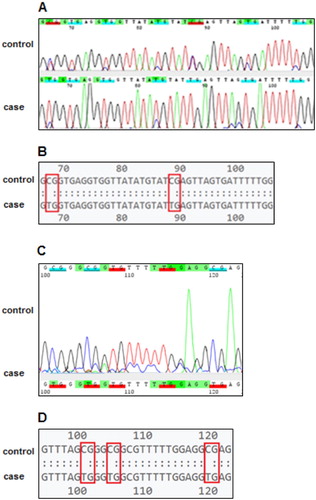
Results of sequence analysis showed an unmethylated pattern for both MMP-2 and MMP-9 in case group. Both genes were partially methylated in control group, while the percent of methylated CpGs was significantly higher in MMP-9 (32%) than MMP-2 (14%) (P = 0.001) ().
Figure 2. Pairwise comparison of target CpG islands between factor XIII deficient patients without ICH (control) and those with ICH (case). (A) Representative chromatogram from Sanger sequencing of the MMP-2 gene. Tags indicate CG and TG dinucleotides. (B) Plain text format from Sanger sequencing of the MMP-2 gene. Boxes indicate CG and TG dinucleotides. (C) Representative chromatogram from Sanger sequencing of the MMP-9 gene. (D) Plain text format from Sanger sequencing of the MMP-9 gene.
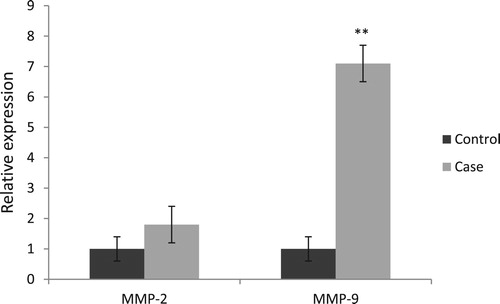
Expression of MMP-2 and MMP-9 at the mRNA level
Our results showed that the MMP-9 (P = 0.008), but no MMP-2 (P = 0.12) gene expression was markedly upregulated in the case group than the control group ().
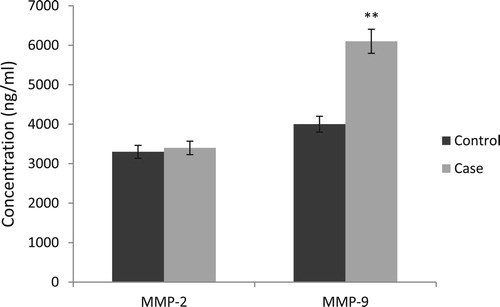
Plasma concentrations of MMP-2 and MMP-9
According to the results, the plasma concentration of MMP-9 was significantly higher in the case group than control group (P = 0.009). In contrast, there was no statistically significant difference in the plasma concentration of MMP-2 of two groups (P = 0.25) ().
Discussion
ICH is the main cause of morbidity and mortality in congenital FXIII deficiency. Due to the rarity of congenital FXIII deficiency, there is a paucity of data in the literature about the pathogenesis and underlying factors of ICH in this disorder [Citation3–6]. According to the previous studies, MMP-2 and MMP-9 with gelatinase activity, have been broadly associated with BBB breakdown and ICH [Citation11,Citation21].
Abilleira et al. for the first time reported overexpression of MMP-9 in human spontaneous ICH. They determined the standard value for the plasma MMP-9 concentration in healthy controls. Our results were in accordance with their study, which indicated that high MMP-9 level in plasma is correlated with ICH [Citation22]. In contrast, Hernandez-Guillamon and colleagues have reported no significant difference in the plasma MMP levels between case and control groups [Citation14].
In a study on patients with congenital FXIII deficiency and ICH it was revealed that MMP-9 mRNA levels were higher in the patients with ICH than the control group [Citation15]. Furthermore, Power et al. [Citation21] provided the evidence of MMP-9 overexpression in an ICH animal model, and other studies have also reported an association between plasma MMP-9 levels and hemorrhagic transformation in human stroke [Citation23,Citation24].
In this study, we found an unmethylated pattern for both MMPs in the case group. In addition, higher expression of MMP-9, but no MMP-2, was found in the case group. Farias et al. [Citation19] in agreement with our findings, showed an unmethylated pattern for MMP-2 and MMP-9, and the higher expression of MMP-9, but no MMP-2, in patients with ameloblastoma. Similar findings were reported by Chicoine, Singh and Roach. They found an inverse correlation between promoter methylation at specific CpG sites and MMP-9 expression at both mRNA and protein levels [Citation17,Citation20,Citation25].
MMP-2 as a constitutive MMP is normally present at high levels in brain tissue and cerebrospinal fluid. Therefore, small changes in the levels are obscured, whereas MMP-9 as an inducible MMP is present at very low concentrations, making it possible to detect small changes. Furthermore, several studies including animal models of cerebral ischemia have reported the dynamic and time-dependent expression of these 2 gelatinases. It is hypothesized that MMP-2 has preferentially role in early opening of the BBB after cerebral ischemia, whereas hemorrhagic transformation in the delayed phase of BBB disruption (After 24–48 h) was associated with elevated MMP-9 expression [Citation21–29]. This could explain our findings concerning the differential expression levels of MMP-2 and MMP-9 in the two groups.
Some limitations of our study should be mentioned. First, small sample size particularly the number of the patients with ICH, due to the rarity of congenital FXIII deficiency. Second, the single blood sample can also affect the results. Third, lack of MMPs activity assays.
In conclusion, our findings support the possibility of measurement of MMP-9 as a prognostic and diagnostic biomarker for ICH. Future investigations are necessary to better understand the regulatory mechanisms of these MMPs and pathogenesis of ICH in congenital FXIII deficiency.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Ali Noroozi-Aghideh http://orcid.org/0000-0002-2811-2659
Zahra Kashani khatib http://orcid.org/0000-0002-6733-203X
Majid Naderi http://orcid.org/0000-0002-4566-8202
Akbar Dorgalaleh http://orcid.org/0000-0002-0125-9319
Marjan Yaghmaie http://orcid.org/0000-0001-7745-9360
Mahdi Paryan http://orcid.org/0000-0002-2592-3528
Shaban Alizadeh http://orcid.org/0000-0001-6253-0822
Additional information
Funding
References
- Dorgalaleh A, Rashidpanah J. Blood coagulation factor XIII and factor XIII deficiency. Blood Rev. 2016;30(6):461–475. doi: 10.1016/j.blre.2016.06.002
- Alavi SER, Jalalvand M, Assadollahi V, et al. Intracranial hemorrhage: a devastating outcome of congenital bleeding disorders – prevalence, diagnosis, and management, with a special focus on congenital factor XIII deficiency. Semin Thromb Hemost. 2018;44(3):267–275. doi: 10.1055/s-0037-1604109
- Dorgalaleh A, Tabibian S, Shams M, et al. A unique factor XIII Mutation in Southeastern Iran with an unexpectedly high prevalence: Khash factor XIII. Semin Thromb Hemost. 2019 Jan 10. doi:10.1055/s-0038-1676580.
- Dorgalaleh A, Alavi SE, Tabibian S, et al. Diagnosis, clinical manifestations and management of rare bleeding disorders in Iran. Hematology. 2017;22(4):224–230. doi: 10.1080/10245332.2016.1263007
- Gheidishahran M, Dorgalaleh A, Tabibian S, et al. Molecular diagnosis of factor XIII deficiency, data from comprehensive coagulation laboratory in Iran. Blood Coagul Fibrinolysis. 2018;29(1):87–91. doi: 10.1097/MBC.0000000000000679
- Naderi M, Ahmadinejad M, Hosseini MS, et al. Long-term prophylaxis in patients with severe congenital factor XIII deficiency is not complicated by inhibitor formation. Blood Coagul Fibrinolysis. 2017;28(4):276–278. doi: 10.1097/MBC.0000000000000578
- Dorgalaleh A, Tabibian S, Sadat Hosseini M, et al. Diagnosis of factor xiii deficiency. Hematology. 2016;21(7):430–439. doi: 10.1080/10245332.2015.1101975
- Dorgalaleh A, Naderi M, Shamsizadeh M. Morbidity and mortality in a large number of Iranian patients with severe congenital factor XIII deficiency. Ann Hematol. 2016;95(3):451–455. doi: 10.1007/s00277-015-2568-8
- Tokito A, Jougasaki M. Matrix metalloproteinases in non-neoplastic disorders. Int J Mol Sci. 2016;17(7):1178. doi: 10.3390/ijms17071178
- Araki Y, Mimura T. Matrix metalloproteinase gene activation resulting from disordred epigenetic mechanisms in rheumatoid arthritis. Int J Mol Sci. 2017;18(5):905. doi: 10.3390/ijms18050905
- Rosenberg GA. Metalloproteinases and neurodegenerative diseases: pathophysiological and therapeutic perspectives. Metalloproteinases Med. 2015;2:39–50. doi: 10.2147/MNM.S68849
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489
- Romanic AM, White RF, Arleth AJ, et al. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats. Stroke. 1998;29:1020–1030. doi: 10.1161/01.STR.29.5.1020
- Hernandez-Guillamon M, Martinez-Saez E, Delgado P, et al. MMP-2/MMP-9 plasma level and brain expression in cerebral amyloid angiopathy-associated hemorrhagic stroke. Brain Pathol. 2012;22(2):133–141. doi: 10.1111/j.1750-3639.2011.00512.x
- Naderi M, Younesi MR, Dorgalaleh A, et al. Association between expression of MMP-2 and MMP-9 genes and pathogenesis of intracranial hemorrhage in severe coagulation factor XIII deficiency. Hematology. 2015;20(8):487–492. doi: 10.1179/1607845414Y.0000000226
- Keep RF, Zhou N, Xiang J, et al. Vascular disruption and blood–brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18
- Chicoine E, Esteve PO, Robledo O, et al. Evidence for the role of promoter methylation in the regulation of MMP-9 gene expression. Biochem Biophys Res Comun. 2002;297:765–772. doi: 10.1016/S0006-291X(02)02283-0
- Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948
- Farias LC, Gomes CC, Rodrigues MC, et al. Epigenetic regulation of matrix metalloproteinase expression in ameloblastoma. BMC Clin Pathol. 2012;12:11. doi: 10.1186/1472-6890-12-11
- Singh K, Agrawal NK, Gupta SK, et al. Differential expression of matrix metalloproteinase-9 gene in wounds of type 2 diabetes mellitus cases with susceptible – 1562 C > T genotypes and wound severity. Int J Low Extrem Wounds. 2014;13(2):94–102. doi: 10.1177/1534734614534980
- Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol. 2003;53:731–742. doi: 10.1002/ana.10553
- Abilleira S, Montaner J, Molina CA, et al. Matrix metalloproteinase – 9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99(1):65–70. doi: 10.3171/jns.2003.99.1.0065
- Montaner J, Alvarez-Sabin J, Molina C, et al. Matrix met-alloproteinase expression is related tohemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2767. doi: 10.1161/hs1201.99512
- Castellanos M, Sobrino T, Mil-lian M, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke. Stroke. 2007;38:1855–1859. doi: 10.1161/STROKEAHA.106.481556
- Roach HI, Yamada N, Cheung KS, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. doi: 10.1002/art.21300
- Alvarez-Sabín J, Delgado P, Abilleira S, et al. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage. Stroke. 2004;35(6):1316–1322. doi: 10.1161/01.STR.0000126827.69286.90
- Silva Y, Leira R, Tejada J, et al. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke. 2005;36:86–91. doi: 10.1161/01.STR.0000149615.51204.0b
- Castellazzi M, Tamborino C, De Santis G, et al. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl. 2010;106:137–140. doi: 10.1007/978-3-211-98811-4_24
- Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7(3):243–253. doi: 10.2174/187152708784936608