ABSTRACT
To systematically evaluate the efficacy and safety of iron chelators for transfusion-dependent patients with MDS. Thirteen cohort studies with 12,990 patients diagnosed with MDS were included in this study. According to m eta-analysis results transfusion-dependent MDS patients with secondary iron overload had a longer (HR = 0.52, 95%CI = 0.43–0.62, P < 0.001). Further subgroup analysis revealed a longer LFS (HR = 0.84, 95%CI = 0.76–0.93, P = 0.001) in MDS patients receiving iron chelators than in MDS patients not receiving iron chelators (HR = 0.52, 95%CI = 0.43–0.62, P < 0.001) and in patients with lower-risk MDS (HR = 0.50, 95%CI = 0.43–0.59, P < 0.001). Subgroup analysis of DFX showed that compared with patients not treated with iron chelators, the group receiving DFX monotherapy had significantly increased OS (HR = 0.43, 95%CI = 0.27–0.69, P < 0.001). In terms of tolerance, meta-analysis of binary variables in CAEs indicated that the occurrence of CAEs was significantly reduced by ICT (RR = 0.64, 95%CI = 0.57–0.71, P < 0.001).
1. Introduction
Myelodysplastic syndrome (MDS) is a group of clonal haematopoietic stem cell diseases that are characterized by haemocytopeania, abnormal development of one or more myeloid lines, ineffective haematopoiesis, and a high risk of leukaemia [Citation1–3]. The incidence of MDS in the population is approximately 5/100,000, and age is an independent risk factor. Furthermore, MDS incidence in the population over 60 years old increases to 20–50/100,000 [Citation4]. The International Prognosis Scoring System (IPSS) of patients, including low risk, intermediate risk 1 and 2 and high risk, is calculated based on the number of primitive cells in the bone marrow, the degree of any decrease in peripheral blood cells and the karyotype [Citation5]. At present, chemotherapy and bone marrow transplantation are the main treatments for intermediate-risk 2 and high-risk patients, whereas transfusion support is the main treatment for low-risk and intermediate-risk 1 patients [Citation4,Citation6]. It has been reported that throughout the course of MDS, approximately 60%–80% of affected patients show symptoms of anaemia and that 80%–90% require erythrocyte infusion as a supportive treatment; transfusion dependence is also very common.
Malcovati et al. [Citation7] conducted a cohort study of 426 patients divided according to WHO subgroups, karyotype abnormalities and transfusion dependence and established the WHO classification Prognostic Score System (WPSS), which classifies MDS patients into five risk groups. These authors also showed that transfusion dependence has an impact on the survival rate and progression of MDS leukaemia among patients in different groups. Anaemia is very common among patients in the course of MDS, with most affected patients suffering from transfusion dependence and life-threatening end-organ damage caused by excessive iron deposits in tissues [Citation8,Citation9]. The guidelines from the National Comprehensive Cancer Network (NCCN) recommend that the population receiving 20–30 units of red blood cells, those receivingongoing red blood cell transfusion, and those with a low or intermediate-risk 1 IPSS score or serum ferritin levels of > 2500 ng/mL, should begin to receive iron chelation therapy (ICT) [Citation10,Citation11]. Moreover, retrospective studies have found that patients with transfusion dependence or serum ferritin >1000 ng/mL, exhibit a much more general pattern of liver or heart dysfunction and worse prognosis [Citation6,Citation12,Citation13]. Therefore, research on ICT in transfusion-dependent MDS patients has been gradually increasing. Currently, the iron chelators mainly used for the clinical treatment of secondary iron overload include Deferasirox (DFX), Deferoxamine (DFO), and Deferiprone (DFP), which reduce transfusion-dependent iron overload mainly by binding to unstable plasma iron to form non-toxic conjugates, which can be excreted from the body through metabolism [Citation14]. Some retrospective studies have also shown a negative correlation with survival for MDS patients who rely on blood transfusions [Citation8,Citation15,Citation16]. However, to the use of iron chelators has remained controversial to date, mainly because of some of the adverse reactions caused by iron chelators or the uncertainty about whether they can effectively increase survival [Citation17]. Therefore, based on the clinical evidence in observational studies, this study integrated and processed clinical data, including overall survival (OS), leukaemia-free survival (LFS), and cardiac adverse events (CAEs), to evaluate the efficacy and safety of iron chelators and their relationship with the survival and prognosis of transfusion-dependent MDS patients. The aim was to provide a basis and ideas for the formulation of clinical treatment schemes and strategies.
2. Methods
2.1. Literature search and inclusion criteria
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [Citation18]. Using retrieval methods in combination with free words and the theme words, we searched the PubMed, Embase, The Cochrane Library, Web of Science, WanFang Data, CNKI and VIP databases for articles published before November 2018. Search terms included transfusion-dependent AND iron overload AND iron chelator (Deferasirox OR Deferomamine OR Deferiprone) AND (myelodysplastic syndrome OR MDS). The references of the retrieved articles were then examined to identify other relevant studies that may meet the inclusion criteria.
The included literature was required to meet the following criteria: (1) cohort studies on the efficacy and safety of iron chelators for transfusion-dependent MDS patients published at home or abroad, including retrospective and prospective studies; (2) all cases involved transfusion-dependent MDS patients with ferritin >1000 mg/L or who received 20–50 units, with a control group of transfusion-dependent MDS patients who did not use iron chelators; (3) similar research methods, diagnostic criteria and intervention measures of each literature among the articles; and (4) complete original data and reasonable statistical methods provided in the study. Studies were excluded if one of the following criteria was fulfilled: (1) case report, review, animal experiment or meeting summary; (2) incomplete original data content ora design scheme lacking rigour; and (3) repeated published studies that used only the largest sample size.
Regarding outcome indicators, the risk ratio (HR) or relative risk (RR) and 95% confidence interval (CI) was used to describe the OS, LFS and CAEs to assessthe survival of patients and efficacy of ICT.
2.2. Data extraction and quality assessment
The literature was sorted independently by 2 researchers according to the inclusion and exclusion criteria, and the following extracted information was selected: the (1) first author, (2) publication time, (3) research type, (4) research population, (5) number of people, (6) number of people in the experimental group, (7) iron chelators, (8) proportions of males and females. The data were summarized and sorted, cross-checked and recorded in a computer database. Any disagreement was resolved by the third researcher.
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included studies [Citation19]. Items included (1) appropriate case determination (1 point), (2) representative cases (1 point), (3) choice of the control (1 point), (4) determination of the control (1 point), (5) considering the comparability of cases and controls in the design and statistical analysis (2 points), (6) exposure factor determination (1 point), (7) using the same method to determine case and control group exposure factors (1 point), and (8) no response rate (1 point). The highest score was 9, with 0–4 indicating low-quality research and 5–9 indicating high-quality research.
2.3. Statistical analysis
All statistical analyses were conducted using Stata SE 15.0 software. For survival data that could not be obtained directly from the article, Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/) was used to construct a Kaplan-Meier curve and calculate the HR and 95% confidence interval. Between-study heterogeneity was evaluated with the I2 statistic. The I2 statistic correlates positively with heterogeneity, and I2 statistics of 25%, 50% and 75% correspond to low, medium and high heterogeneity, respectively. If I2 < 50% or P > 0.1, the heterogeneity between the studies was considered to be small, and the fixed effects model (FEM) was selected for data consolidation. In contrast, if the heterogeneity between the studies was considered to be relatively large, we analyzed the source of heterogeneity, performed a subgroup analysis and sensitivity analysis, and used a random-effects model (REM) if heterogeneity still existed. P values ≤0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the included studies
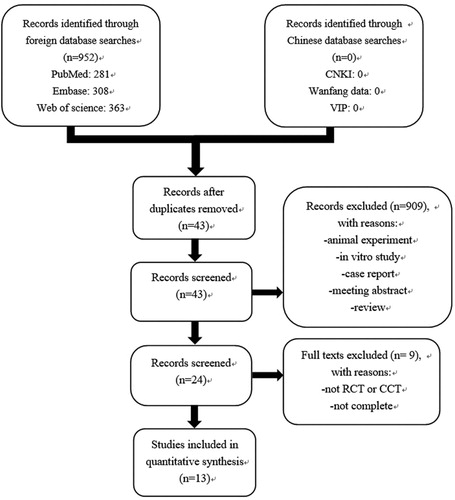
Initially, 952 articles (see ) were obtained via database retrieval. After excluding irrelevant articles by re-reading the titles, abstracts and content data, 13 cohort studies were ultimately evaluated; including 13,000 MDS patients, 7236 of whom were men (55.1%). The basic characteristics described in the literature were as follows (see ). Among the total study patients, there were 1999 patients who received iron chelators, 11,001 MDS patients who did not receive iron chelators, and 1865 MDS patients with lower risk (LR) on IPSS, of whom 809 received iron chelators and 1056 did not receive iron chelators.
Table 1. Baseline characteristics of the included studies.
3.2. Literature quality evaluation and methods
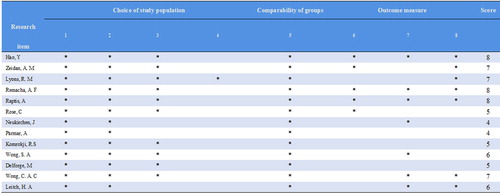
Two different researchers evaluated the titles and abstracts of the 952 relevant articles, screened out the articles that might meet the inclusion criteria, further analyzed the data and contents of the full text, and assessed the included articles according to the inclusion and exclusion criteria. Two researchers cross-checked and cross-evaluated the results. If there was any disagreement regarding an article, the third researcher decided whether to include it. The included articles were graded according to the NOS evaluation criteria. Three of the 13 studies included were prospective studies. After NOS quality evaluation, 11 were considered high-quality articles, and 2 were considered low-quality articles. Therefore, this study is highly reliable as a whole ().
Figure 2. NOS quality evaluation of the included articles.
Note: 1 Representative of the exposure group. 2 Selection method of the non-exposure group. 3 Determination of exposure factors. 4 Determination of the outcome index that had not been observed at the beginning of the study. 5 Considering the comparability between the exposure group and the non-exposure group during the statistics and statistical analysis. 6 Whether the evaluation of the results in the study was adequate. 7 Whether the follow-up was long enough after the obtaining results. 8 Whether the follow-up of the exposure and non-exposure groups was sufficient.
4. Data analysis
4.1. Correlation analysis of ICT and OS in MDS patients
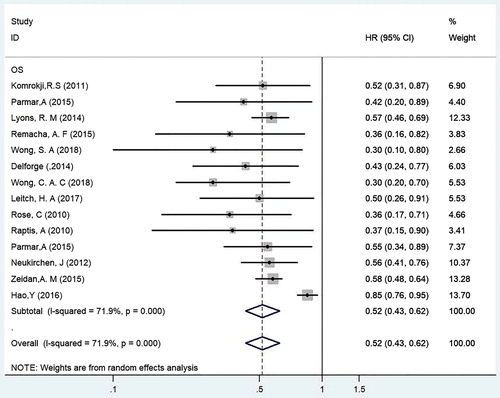
Thirteen cohort studies involving 12,990 MDS patients, 1999 of whom received iron chelators and the remainder were not treated with iron chelators, were included in this meta-analysis. The HR and 95%CI of OS were analyzed for MDS patients treated and not treated with iron chelators (see ) [Citation20–32]. The results of the included studies were tested for heterogeneity, which indicated that heterogeneity existed among the studies (P < 0.001, I2 = 71.9%). Therefore, the REM was used for the analysis. The HR of OS in MDS patients who received iron chelators and those who did not receive iron chelators was 0.52 (95%CI = 0.43–0.62, P < 0.001). The results showed that ICT significantly enhanced the OS of MDS patients and that receiving iron chelators was an effective clinical treatment in transfusion-dependent MDS patients.
4.2. Correlation analysis of ICT and OS in patients with low-risk (LR) MDS by IPSS typing
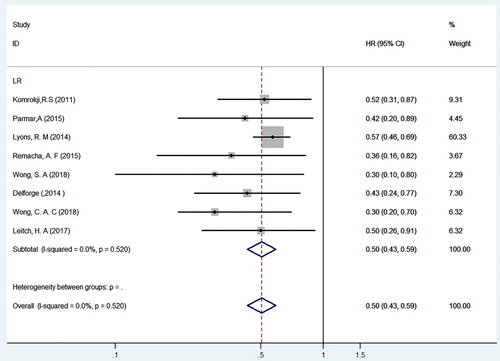
Subgroup analysis for the correlation between ICT and OS in MDS patients with a lower risk by IPSS typing was performed (see ). The subgroup analysis included 8 studies that involved 1865 patients with lower-risk MDS, 809 of whom received iron chelators, with the remained not receiving iron chelators [Citation22,Citation23,Citation27–32]. Because there was no heterogeneity among the included studies (P = 0.520, I2 = 0.0%), correlation analysis of the subgroups was conducted using FEM. The HR of OS for low-risk MDS patients treated with or without iron chelators was 0.50 (95%CI = 0.43–0.59, P < 0.001). Therefore, it is clear that lower-risk MDS patients, including those with low-risk and intermediate-risk 1 transfusion-dependent in IPSS typing, significantly benefit from iron chelators, which improved their survival status and effectively improved their OS. For patients with lower-risk MDS who had received long-term transfusion treatment clinically, it is recommended that the treatment should be used when they meet the conditions for ICT.
4.3. Correlation analysis between ICT and LFS in MDS patients
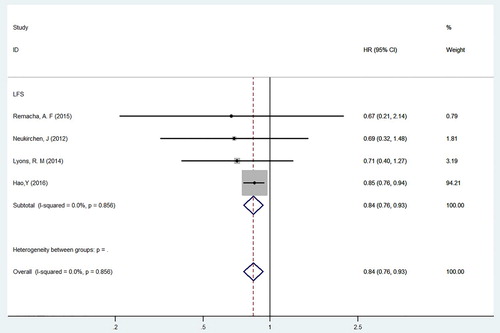
The subgroup analysis included 4 cohort studies involving 7848 MDS patients, with 4529 males and 3319 females; of these, 1094 MDS patients received iron chelators (see ) [Citation20,Citation22,Citation23,Citation26]. A heterogeneity test was carried out on the 4 included studies, and the results showed little difference in heterogeneity among the studies (P = 0.856, I2 = 0.0%); therefore, FEM was used. After an integrated analysis of the data, the HR of LFS for MDS patients treated with and without iron chelators was 0.84 (95%CI = 0.76–0.93, P = 0.001). This suggests that receiving iron chelators may reduce the risk of leukaemia transformation in long-term transfusion-dependent MDS patients.
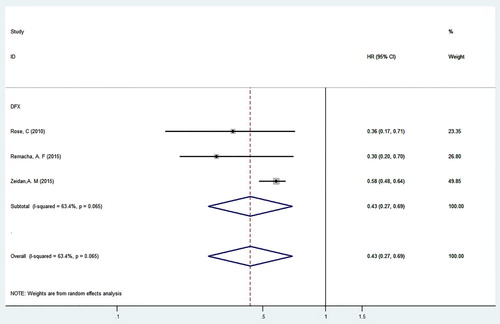
4.4. Correlation analysis between DFX monotherapy and OS in MDS patients
Three of the included studies involved MDS patients treated with a single drug of that was the new oral iron chelator DFX (see ). There were 4286 patients, including 2205 males and 1777 females; of these, 597 MDS patients were treated with ICT. A heterogeneity test was conducted, and the results showed that there was heterogeneity among the studies (P = 0.065, I2 = 63.4%); therefore, REM was used. According to the results of the integration analysis, HR of OS in MDS patients receiving DFX monotherapy and in those not receiving iron chelators was 0.43 (95%CI = 0.27–0.69, P < 0.001). Therefore, DFX significantly improved OS in transfusion-dependent MDS patients. This analysis also provides a meaningful reference for drug regimens for transfusion-dependent MDS patients.
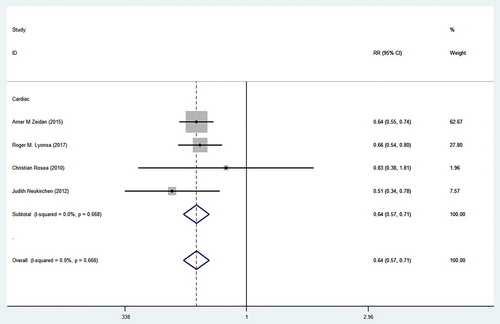
4.5. Meta-analysis of CAEs in MDS patients treated with iron chelators
A total of 4811 MDS patients were included in the 4 studies, 808 and 4003 of whom received and did not receive iron chelators respectively (see ). The four studies extracted target data and then synthesized the relative risk ratio (RR) for analysis. The heterogeneity analysis indicated that the four studies were homogeneous (P = 0.668, I2 = 0.0%), and a FEM was therefore used. After the integration of the 4 studies, the CAEs among MDS patients who received iron chelators and those who did not receive iron chelators were found to have a relative risk of 64% (95%CI = 57% to 71%, P < 0.001).Hence, we suggest that iron chelators exert a significant effect by reducing the incidence of CAEs in patients with chronic transfusion-dependent MDS.
5. Discussion
The rationale for ICT in transfusion-dependent MDS patients is compelling but has not been evaluated in prospective randomized trials. In this study, a meta-analysis of relevant articles and clarify the effect of iron chelators on the survival prognosis of transfusion-dependent MDS. MDS patients may have iron overload before transfusions, mainly due to ineffective erythropoiesis and/or increasing intestinal iron absorption [Citation33]. However, studies have shown that the main cause of iron overload in MDS patients is chronic transfusion therapy because each unit of red blood cells contains 100–250 mg of iron, and transfusion dependence has been defined as ‘high transfusion burden’ or ‘low transfusion burden’ depending on the number of units transfused over a given time period, usually 8 or 16 weeks. [Citation34,Citation35]. Because the human body lacks iron excretion mechanisms, long-term chronic transfusion leads to secondary iron overload. Although iron overload does not cause obvious symptoms in patients in the shortterm, without treatment, secondary iron overload usually causes progressive damage to the liver, endocrine organs and heart organs in the long term, seriously affecting the quality of life and survival time of affected patients [Citation3,Citation36,Citation37]. To date, the organ dysfunction caused by iron overload and the benefits from ICT have been intensively studied in thalassaemia and other transfusion-related anaemias, but clinical studies of MDS are relatively scarce, and this patient group has not been clearly evaluated in large RCTs [Citation9,Citation38–40]. Compared with thalassaemia patients, MDS patients are older, have poorer physical functions and may suffer from various chronic diseases, rendering them more sensitive to iron overload [Citation41].In the interim, the empirical evidence in support of ICT intransfusion-dependent MDS patients has come from observational studies [Citation34].
Through collection of relevant included articles, we first found that although some studies have performed a survival analysis of transfusion-dependent MDS patients, none were in-depth analyses, and they merely covered OS and single or unstratified variables. Second, we found that the OR or RR was sometimes used to analyze the effect scale, whereby the authors simply used the ratio of the incidence of survival rates between the two groups at a fixed point and did not consider all the factors into account; hence, the results did not describe the entire scale of the data, and the conclusions drawn from such incomplete data are unreliable. These approaches required us to use a measure that would reflect the results of the occurrence of an event and the timing of the occurrence of the event (i.e. HR) [Citation42,Citation43]. The initial development of iron chelators as a treatment for transfusion-dependent MDS was initiated by a research centre in France who found that iron overload had a negative impact on the morbidity and mortality of MDS patients and that iron chelators had a beneficial impact on clinical results in low- and intermediate-risk MDS patients [Citation13]. A recent multicentre prospective study reported a the median OS of 115 months for 165 MDS patients treated with iron chelators and 51 months for MDS patients not treated with iron chelators (P < 0.001) [Citation25]. In a prospective study of 239 patients with transfusion-dependent MDS, Leitch et al. [Citation32] found that 89 of 239 MDS patients with transfusion dependence received iron chelators and that the median OS was significantly better in the ICT group than in the group who did not receive iron chelators (5.2 vs 2.1 years, P < 0.0001). Multivariate analysis also showed that not being treated with iron chelators was a poor prognostic factor for transfusion-dependent MDS patients (P = 0.03) but that the median OS of patients treated with iron chelators was significantly longer (P = 0.02), further demonstrating that ICT may have additional benefits in clinical practice. According to specific criteria and our evaluation of the literature, we included 13 articles in this study. A quantitative analysis of the included literature showed that iron chelators may provide survival benefits for MDS patients in terms of extending survival times (HR = 0.52, 95%CI = 0.43–0.62, P < 0.001). Even though the paper reports a statistically significant difference in OS between chelated and non-chelated patients (HR = 0.52, 95%CI = 0.43–0.62, p < 0.001). While there was also heterogeneity existed among chelated and non-chelated patients (P < 0.05). As our review was focused on lower-risk MDS patients, because the chelated patients would tend to be from low risk studies and the non-chelated would tend to be from higher risk studies. As we performed that only 15% of the approximately 13,000 MDS patients were chelated. This may reflect clinician reluctance, especially in the absence of RCT efficacy data, to treat such patients routinely with ICT. It may also suggest patient-specific clinical decision-making. In summary, the overall usefulness of this initial cohort analysis is limited for clinical practice.
Approximately 20% of patients with MDS will progress to acute myeloid leukaemia (AML) [Citation1]. Based on existing clinical studies, there is some controversy regarding whether iron chelators have an effect on AML transformation. In a retrospective study, paired analysis of registered MDS patients showed that although iron chelators resulted in a longer OS, there was no significant difference in terms of the risk of leukaemia transformation [Citation26]. Regardless, some studies have shown that iron chelators can prolong the transformation time of leukaemia. A meta-analysis of the survival indexes of transfusion iron overload in MDS patients treated with iron chelators showed that iron overload caused by red blood cell transfusion was an independent adverse prognostic factor affecting OS and LFS, even though transfusion improved quality of life and reduced the risk of leukaemia transformation (HR = 0.84, 95%CI = 0.76–0.93, P = 0.001). Further subgroup analysis showed that ICT also have a significant effect on low-risk MDS patients and significantly prolonged their OS (HR = 0.50, 95%CI = 0.43–0.59, P < 0.001). DFX, a new oral iron chelator, had been approved by the FDA and EMA for the treatment of secondary iron overload. It has a half-life of 8–16 h and is orally administered once a day. DFX is more acceptable for the general patient; in addition, it is mainly excreted from the body via faeces and is therefore less harmful to organs; thus, DFX attracted our attention [Citation44]. In the present, we compared the group receiving DFX as a monotherapy iron chelator with the group not receiving DFX and found that the OS of transfusion-dependent MDS patients was improved by DFX (HR = 0.43, 95%CI = 0.27–0.69, P < 0.001). During treatment with an iron chelator, adverse events, such as cardiac insufficiency, impaired liver function and gastrointestinal reactions, may occur; among these, the main cause of death is CAEs [Citation45]. However, in terms of heart risk, it is difficult to determine how iron chelation provides a survival advantage for MDS patients. According to data from the American Registry,the mortality rate of heart disease was lower, the frequency of infection was lower, and other malignant tumours were very rare in the ICT group, consistent with the conclusions presented herein (RR = 64%, 95%CI = 57% to 71%, P < 0.001) [Citation22].
This systematic review and meta-analysis included thirteen studies involving 12,990 patients. Most of the studies (11/13) were high-quality and included NOS scores. Iron chelators were found to provide survival benefits for MDS patients with transfusion dependence, mainly in terms of prolonging OS and LFS, and with relatively good cardiac tolerance. There are some limitations to this study. For example, the number of studies included was relatively small, and most were retrospective. However, the criteria for transfusion dependence varied among the groups due to the limited original data, and it was impossible to conduct a stratified analysis of the categories and doses of other iron chelators or further subgroup analysis according to the severity of the disease. Therefore, the influence of ICT on the survival and prognosis of transfusion-dependent MDS patients still needs to be verified in large-sample, multi-centre, double-blind prospective randomized control studies to provide more ideas and directions for clinical work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adès L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383:2239–2252. doi: 10.1016/S0140-6736(13)61901-7
- Jabbour E, Kantarjian HM, Koller C, et al. Red blood cell transfusions and iron overload in the treatment of patients with myelodysplastic syndromes. Cancer. 2008;112:1089–1095. doi: 10.1002/cncr.23280
- Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038
- Cazzola M, Malcovati L. Myelodysplastic syndromes — coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536–538. doi: 10.1056/NEJMp048266
- Della Porta MG, Tuechler H, Malcovati L, et al. Validation of WHO classification-based Prognostic Scoring System (WPSS) for myelodysplastic syndromes and comparison with the revised International Prognostic Scoring System (IPSS-R). A study of the International Working Group for Prognosis in Myelodysplasia (IWG-PM). Leukemia. 2015;29:1502–1513. doi: 10.1038/leu.2015.55
- Killick SB, Carter C, Culligan D, et al. Guidelines for the diagnosis and management of adult myelodysplastic syndromes. Br J Haematol. 2014;164:503–525. doi: 10.1111/bjh.12694
- Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696
- Temraz S, Santini V, Musallam K, et al. Iron overload and chelation therapy in myelodysplastic syndromes. Crit Rev Oncol Hematol. 2014;91:64–73. doi: 10.1016/j.critrevonc.2014.01.006
- Porter JB. A risk-benefit assessment of iron-chelation therapy. Drug Saf. 1997;17:407–421. doi: 10.2165/00002018-199717060-00006
- Greenberg PL, Rigsby CK, Stone RM, et al. NCCN task force: transfusion and iron overload in patients with myelodysplastic syndromes. J Natl Compr Canc Netw. 2009;7(Suppl. 9):S1–S16. doi: 10.6004/jnccn.2009.0082
- Bennett JM, MDS Foundation's Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83:858–861. doi: 10.1002/ajh.21269
- Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol. 2007;78:487–494. doi: 10.1111/j.1600-0609.2007.00842.x
- Leitch HA. Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res. 2007;31:S7–S9. doi: 10.1016/S0145-2126(07)70460-5
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546–4551. doi: 10.1182/blood-2009-05-224188
- Cermak J, Kacirkova P, Mikulenkova D, et al. Impact of transfusion dependency on survival in patients with early myelodysplastic syndrome without excess of blasts. Leuk Res. 2009;33:1469–1474. doi: 10.1016/j.leukres.2009.06.033
- Malcovati L. Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk Res. 2007;31(Suppl 3):S2–S6. doi: 10.1016/S0145-2126(07)70459-9
- Neukirchen J. Iron chelation in MDS: still a controversial issue. Leuk Res. 2014;38:145–146. doi: 10.1016/j.leukres.2013.12.004
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z
- Hao Y, Fu AZ, Coe A, et al. Clinical outcomes among patients with myelodysplastic syndromes treated with iron chelation therapy: a real world medicare database study. Blood. 2016;128:2396–2396.
- Zeidan AM, Hendrick F, Friedmann E, et al. Deferasirox therapy is associated with reduced mortality risk in a medicare population with myelodysplastic syndromes. J Comp Eff Res. 2015;4:327–340. doi: 10.2217/cer.15.20
- Lyons RM, Marek BJ, Paley C, et al. Relation between chelation and clinical outcomes in lower-risk patients with myelodysplastic syndromes: Registry analysis at 5 years. Leuk Res. 2017;56:88–95. doi: 10.1016/j.leukres.2017.01.033
- Remacha AF, Arrizabalaga B, Villegas A, et al. Evolution of iron overload in patients with low-risk myelodysplastic syndrome: iron chelation therapy and organ complications. Ann Hematol. 2015;94:779–787. doi: 10.1007/s00277-014-2274-y
- Raptis A, Duh MS, Wang ST, et al. Treatment of transfusional iron overload in patients with myelodysplastic syndrome or severe anemia: data from multicenter clinical practices. Transfusion. 2010;50:190–199. doi: 10.1111/j.1537-2995.2009.02361.x
- Rose C, Brechignac S, Vassilief D, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myelodysplasies). Leuk Res. 2010;34:864–870. doi: 10.1016/j.leukres.2009.12.004
- Neukirchen J, Fox F, Kundgen A, et al. Improved survival in MDS patients receiving iron chelation therapy - a matched pair analysis of 188 patients from the Dusseldorf MDS registry. Leuk Res. 2012;36:1067–1070. doi: 10.1016/j.leukres.2012.04.006
- Parmar A, Leitch HA, Wells RA, et al. Iron chelation is associated with improved survival adjusting for disease and patient related characteristics in Low/Int-1 risk MDS at the time of first transfusion dependence: a MDS-CAN study. Blood. 2015;126:1701–1701.
- Komrokji RS, Ali NHA, Padron E, et al. Impact of iron chelation therapy on overall survival and AML transformation in lower risk MDS patients treated at the Moffitt Cancer Center. Blood. 2011;118:2776–2776.
- Wong SA, Leitch HA. Iron chelation therapy in lower IPSS risk myelodysplastic syndromes; which subtypes benefit? Leuk Res. 2018;64:24–29. doi: 10.1016/j.leukres.2017.11.005
- Delforge M, Selleslag D, Beguin Y, et al. Adequate iron chelation therapy for at least six months improves survival in transfusion-dependent patients with lower risk myelodysplastic syndromes. Leuk Res. 2014;38:557–563. doi: 10.1016/j.leukres.2014.02.003
- Wong CAC, Wong SAY, Leitch HA. Iron overload in lower international prognostic scoring system risk patients with myelodysplastic syndrome receiving red blood cell transfusions: Relation to infections and possible benefit of iron chelation therapy. Leuk Res. 2018;67:75–81. doi: 10.1016/j.leukres.2018.02.005
- Leitch HA, Parmar A, Wells RA, et al. Overall survival in lower IPSS risk MDS by receipt of iron chelation therapy, adjusting for patient-related factors and measuring from time of first red blood cell transfusion dependence: an MDS-CAN analysis. Br J Haematol. 2017;179:83–97. doi: 10.1111/bjh.14825
- Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348–359. doi: 10.1056/NEJMra1004967
- Gattermann N. Iron overload in myelodysplastic syndromes (MDS). Int J Hematol. 2018;107:55–63. doi: 10.1007/s12185-017-2367-1
- Abraham I, Yami MA, Yun S, et al. Survival outcomes in iron chelated and non-chelated patients with lower-risk myelodysplastic syndromes: review and pooled analysis of observational studies. Leuk Res. 2017;57:104–108. doi: 10.1016/j.leukres.2017.03.007
- Porter JB. Practical management of iron overload. Br J Haematol. 2001;115:239–252. doi: 10.1046/j.1365-2141.2001.03195.x
- Payne KA, Rofail D, Baladi JF, et al. Iron chelation therapy: clinical effectiveness, economic burden and quality of life in patients with iron overload. Adv Ther. 2008;25:725–742. doi: 10.1007/s12325-008-0085-z
- Bou-Fakhredin R, Bazarbachi AH, Chaya B, et al. Iron overload and chelation therapy in non-transfusion dependent thalassemia. Int J Mol Sci. 2017;18:2778–2786. doi: 10.3390/ijms18122778
- Borgna-Pignatti C, Marsella M. Iron chelation in thalassemia major. Clin Ther. 2015;37:2866–2877. doi: 10.1016/j.clinthera.2015.10.001
- Meerpohl JJ, Antes G, Rücker G, et al. Deferasirox for managing iron overload in people with myelodysplastic syndrome. Cochrane Database Syst Rev. 2010;10:1–39.
- Greenberg PL. Myelodysplastic syndromes: iron overload consequences and current chelating therapies. J Natl Compr Canc Netw. 2006;4:91–96. doi: 10.6004/jnccn.2006.0010
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16
- Spruance SL, Reid JE, Grace M, et al. Hazard ratio in clinical trials. Antimicrob Agents Chemother. 2004;48:2787–2792. doi: 10.1128/AAC.48.8.2787-2792.2004
- Messa E, Carturan S, Maffe C, et al. Deferasirox is a powerful NF-kappaB inhibitor in myelodysplastic cells and in leukemia cell lines acting independently from cell iron deprivation by chelation and reactive oxygen species scavenging. Haematologica. 2010;95:1308–1316. doi: 10.3324/haematol.2009.016824
- Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858