ABSTRACT
Objectives:: To investigate the efficacy and safety of second-line treatment in Thai patients with primary warm-type autoimmune hemolytic anemia (AIHA) that failed corticosteroid treatment.
Methods:: This descriptive retrospective study included patients aged >14 years who were diagnosed with and treated for primary warm-type AIHA at the Division of Hematology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, during January 2007 to December 2016. All 54 included patients failed first-line corticosteroid treatment after which second-line treatment was prescribed. Baseline clinical characteristics, laboratory results at diagnosis and at start of second-line treatment, type of second-line treatment, treatment outcome, and complications of treatment including death were collected.
Results:: Included patients had a mean age at onset of 55.8 years (14.5–87.4) and 83.3% of patients were female. Most patients (63%) were refractory to steroids, and the rest of them relapsed while on steroids. The second-line medications were azathioprine (61.1%), cyclophosphamide (31.5%), chlorambucil (1.9%), danazol (3.7%), and rituximab (1.9%), with respective response rates of 78.8%, 58.8%, 1/1 patient, 2/2 patients, and 0/1 patient. Strong positive direct Coombs’ test (3+–4+) was the only predictive factor of treatment response (p = 0.008). Males had better relapse-free survival than females (not reached vs. 20.6 months) (p = 0.023). Approximately 40% of the patients who responded to second-line treatment relapsed at a median of 7.4 months.
Conclusion:: Immunosuppressive drugs are the most common second-line treatment for primary warm-type AIHA in Thailand; however, relapse was common. Additional therapies are needed to reduce the relapse rate and prolong remission.
Introduction
Autoimmune Hemolytic Anemia (AIHA) is rare, having an estimated annual incidence 1–3 cases per 100,000 individuals [Citation1]. In general, it can be classified into warm- (37°C), cold- (4°C), and mixed warm-and-cold-type AIHAs, based on the temperature at which autoantibodies are able to optimally attach to red blood cells (RBCs) [Citation2,Citation3]. Clinical manifestation, serological characteristics, and treatment of choice are distinct for each type: for example, for warm-type AIHA, steroids constitute the first-line treatment, and are associated with a high response rate [Citation3], whereas most patients with cold-type AIHA require only avoidance of cold exposure and they have little or no response to steroids [Citation2,Citation4]. AIHA can also be divided clinically into primary and secondary disorders, depending on the absence or presence of underlying diseases [Citation5] (such as systemic lupus erythematosus, lymphoproliferative diseases, solid malignancies, and drugs), respectively. Treatment options and prognosis for secondary AIHA mainly depend on the associated disease, whereas primary AIHA, which is treated by corticosteroids or non-corticosteroids immunosuppressive agents, has an unpredictable disease course [Citation6].
In this study, we focused on warm-type AIHA because it is the most common (70–80%) AIHA [Citation2,Citation3]. Whereas cold- and mixed-type, which have different clinical characteristics and serological findings from those of warm-type AIHA, account for 15–20% [Citation2] and 7–8% [Citation7,Citation8] of all AIHAs, respectively. Moreover, patients with secondary AIHA were excluded from the study according to their heterogeneous treatment regimens and prognosis. Because AIHA is relatively rare, information of treatments for primary warm-type AIHA are scarce based on retrospective studies with small sample sizes and expert opinions [Citation9–13]. No single drug is known to effectively treat and maintain long-term remission of the disease. Although systemic corticosteroid is the first-line treatment of primary warm-type AIHA on account of its very high response rate (75–97%) [Citation14,Citation15], it leads to permanent complete remissions in only two thirds of cases [Citation4]. Consequently, 27–45% of patients need second-line treatment [Citation15,Citation16], for which there are many options: immunosuppressive drugs (e.g. cyclophosphamide, azathioprine, chlorambucil, and cyclosporine), danazol, rituximab, and splenectomy. While responses have generally been described in a few studies done in Caucasians, there is much less information in Asian populations regarding second-line treatment. Consequently, most physicians in Asia choose second-line treatment based on the existing data on Caucasian studies and on their personal experience.
We hypothesized that a retrospective study which focuses on the efficacy and safety of second-line treatment in primary warm-type AIHA will yield a better picture of drug response, and will help clinicians make decisions when they need to choose such treatment. To this end, here we have followed Thai patients with primary warm-type AIHA at Siriraj Hospital, a tertiary-care national referral center.
Materials and methods
Study design
This was a descriptive, retrospective non-interventional study of patients with primary warm-type AIHA who were treated with second-line treatment at the Division of Hematology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. The study was approved by the Siriraj Institutional Review Board (SIRB) COA no. Si394/2016, and the committee waived the requirement to obtain consent for the retrospective collection, analysis, and publication of the anonymized data.
Patients
Data from 54 patients with primary warm-type AIHA – who were refractory or relapsed from steroids – were collected consecutively, between January 1, 2007, and December 31, 2016. Inclusion criteria were: patients age of more than 14 years; patients diagnosed with warm-type AIHA by hematologists at Siriraj Hospital, using the following criteria: new onset anemia, no cold-induced symptoms (e.g. acrocyanosis), and presence of microspherocytes without erythrocyte agglutination (clumping of individual's RBCs) on peripheral blood smear, with or without positive result of polyspecific direct Coombs’ test, in addition to the absence of any associated diseases (e.g. autoimmune diseases, lymphoproliferative diseases, solid malignancies, infection, and drugs); patients had received at least 1 regimen of second-line therapy, such as immunosuppressive drugs, rituximab, splenectomy, or others. Excluded were patients who had been followed for less than 6 months after receiving second-line therapy, to ensure that every participant received the full effect of treatment response [Citation17]. The medical record of each patient was reviewed for baseline clinical characteristics, laboratory results at diagnosis and at the start of the second-line treatment, type of second-line treatment, treatment outcome, and complications of treatment including death.
Terminology
The definitions of treatment response for both first-line and second-line treatments were adopted from Barcellini et al. [Citation14]. Complete response (CR) was defined as hemoglobin (Hb) ≥12 g/dL and normalization of all serological markers of hemolysis. Partial response (PR) was defined as Hb ≥10 g/dL or ≥2 g/dL increase in Hb from baseline without the need for transfusion. Overall response rate was defined as the combination of the PR rate and the CR rate. Relapse was defined as Hb ≤10 g/dL or ≥2 g/dL decrease in Hb after PR or CR. Refractory disease was defined as persistent Hb <10 g/dL or >2 g/dL decrease in Hb since starting treatment. Responsive patient was defined as a patient who achieved PR or CR after treatment with second-line treatment. Non-responsive patient was defined as a patient who could not achieve at least PR after second-line therapy. Time to response was defined as the duration from initiation of second-line treatment to the time of achieving at least PR. Time to relapse was defined as the duration from achieving at least PR until relapse. Relapse-free survival (RFS) was defined as the duration from the date of achieving PR or CR from second-line treatment to relapse, death, or last follow-up – whichever occurred first.
Statistical analysis
Statistical analysis was performed using PASW Statistics 18.0 (SPSS, Inc., Chicago, IL, USA). Results were shown as fraction, frequency and percentage, mean or median, minimum, and maximum. Mann–Whitney U test was used to compare continuous variables between groups. Chi-square test or Fisher's exact test was used to compare categorical variables. Univariate and multivariate logistic regression models were used to determine variables which can predict response or non-response outcome and calculate odds ratios (OR) with 95% confidence intervals (CI). Kaplan–Meier analysis with log-rank test was used to compare survival between groups. Multiple regression analysis was used to identify variables that can predict relapse-free survival (RFS). A two-sided p-value less than 0.05 was regarded as being statistically significant.
Results
Baseline characteristics at diagnosis and outcome of first-line treatment
Mean age at onset was 55.8 years (14.5–87.4), and most patients were female (83.3%). Initial Hb levels were <6 g/dL in 42.6%, 6–7.9 g/dL in 42.6%, and 8–10 g/dL in 11.1%. At diagnosis, 14.8% of patients had hepatomegaly, and a similar percentage had splenomegaly. All patients in this study were treated with prednisolone as a first-line treatment. Most patients (63%) were refractory to first-line treatment, and 13% and 24.1% achieved PR and CR, respectively. The median time to relapse was 36.3 months (2.2–381).
Baseline characteristics at initiation of second-line treatment
The mean age of patients was 58.2 years (17–87.4), and the majority (63%) received second-line treatment due to being refractory to steroids. Sixty percent of patients with relapsed AIHA were started second-line drugs at Hb range of 8–10 g/dL. Approximately 92% of patients had a positive direct Coombs’ test. Other biochemical results are shown in . Percentage of patients, with active hemolysis, who had normal range of biochemical results were 19.2% in absolute reticulocyte count, 25.6% in lactate dehydrogenase (LDH), 43.8% in total bilirubin, and 78% in aspartate aminotransferase.
Table 1. Clinical and biological characteristics at initiation of second-line treatment.
Efficacy and safety of second-line treatment
Almost all of the patients that received second-line therapy were treated with immunosuppressive drugs, including azathioprine (61.1%), cyclophosphamide (31.5%), and chlorambucil (1.9%). A small proportion of patients were treated with rituximab (1.9%) or danazol (3.7%). Splenectomy was not performed in any study patient. No patients were treated with combination drug therapy. Among those who received second-line treatment, 72.2% responded to treatment. Of those, 35.2% had a complete response, and 37% had a partial response. However, 38.5% of patients that responded to second-line treatment relapsed. The response rate, time to response, relapse rate, and time to relapse for each drug is shown in .
Table 2. Efficacy of second-line treatment.
Second-line therapy was accompanied by considerable side effects that included infection (37%), thrombosis (5.6%), bone marrow suppression (5.6%), and death (5.6%) (). For patients who received azathioprine, 9.1% of them died due to concomitant infection. The 3 thrombotic events, all of which occurred in patients that took azathioprine, were pulmonary embolism, deep venous thrombosis, and cerebral venous sinus thrombosis.
Table 3. Drug-related side effects and adverse reactions.
Factors associated with overall response to second-line treatment
The results of analysis showed that only two variables were significantly different between response and non-response groups. Strong positive direct Coombs’ test (3+–4+) was found in 81.1% of response patients, whereas it was found in 33.3% of non-response patients (p = 0.002). Median LDH of 725 U/L (303–2,730) was found in response group, whereas median LDH of 518 U/L (378–910 U/L) was found in non-response group (p = 0.028). Univariate and multivariate logistic regression analyses showed only strong positive direct Coombs’ test to be significantly associated with increased probability of response to second-line medication (p = 0.008, OR: 11.62, 95% CI: 1.9–71.14).
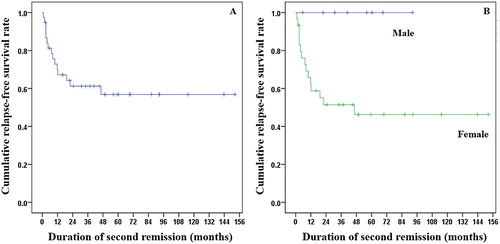
Kaplan–Meier survival analysis of RFS after second-line therapy is shown in (A). The 1-year and 2-year RFS rates were 70% and 61.2%, respectively. Comparing RFS between groups, only gender showed statistically significant difference. Men had better RFS (median RFS: not reached) than women (median RFS: 20.6 months) (p = 0.023) (B). All eight male patients who responded from second-line medication did not relapse or die at the end of study, whereas 12 and 3 out of 30 female patients relapsed and died, respectively. In this analysis, factors thought to predict RFS, including age group, gender, results of direct Coombs’ test, different LDH ranges, and type of medication, were assessed; no significant factor can be used to predict RFS in this cohort.
Figure 1. Kaplan–Meier survival analysis shows the RFS of patients with primary warm-type autoimmune hemolytic anemia after second-line treatment. (A) 1-year RFS was 70%, and 2-year RFS was 61.2%. (B) RFS according to gender, males had better RFS than females (median RFS: not reached vs. median RFS: 20.6 months) (p = 0.023).
Discussion
The results of this study revealed a 70% response rate from second-line treatment in primary warm-type AIHA patients who relapsed during or were refractory to steroid therapy that was given as a first-line treatment, and this finding is consistent those of previous studies [Citation17,Citation18]. These results more or less reflect the response rate to azathioprine or cyclophosphamide therapy since one or the other of these two drugs was used in approximately 90% of cases in this study. Furthermore, strong positive direct Coombs’ test before starting second-line treatment was the only predictive factor of treatment response, and this finding has never been previously reported. However, approximately 40% of patients who responded to second-line treatment relapsed within 1 year, which suggests that the evaluated second-line treatments do not deliver a sustained response.
On the other hand, there are differences between our results and those reported from previous studies. Compared to our study, Barcellini et al. [Citation14] and Rattarittamrong et al. [Citation15] reported the higher response rates from immunosuppressive drugs of approximately 80% and 90%, respectively. In both of those studies, azathioprine and cyclophosphamide were the two most commonly used immunosuppressive drugs. Most patients in those two studies received immunosuppressants early as a steroid-sparing agent. In contrast, all of our patients were treated with immunosuppressants after they demonstrated steroid treatment failure. Rattarittamrong et al. reported that 60% of patients received second-line treatment due to steroid dependence (inability to reduce prednisolone to less than 15 mg/day to maintain Hb level at least 10 g/dL), i.e. their Hb level reached ≥10 g/dL when they were treated. Whereas all of our relapsed patients received second-line treatment when their Hb level was ≤10 g/dL. Barcellini et al. reported that 13% of patients that responded to second-line treatment relapsed, while nearly 40% of our responsive patients relapsed. The disappointing outcomes from our study may be due to a delay in the start of second-line treatment. However, our study seems to reflect the true efficacy of second-line treatment, because all of the patients that we included had demonstrated steroid treatment failure. These findings can be used to guide clinicians regarding the use of immunosuppressants as steroid-sparing agents that should be started early, because it takes months before onset of response. However, patients should be advised of the high possibility of relapse in the relatively near future.
Splenectomy has also been indicated as a second-line therapy in various hematologic disorders [Citation19,Citation20], including AIHA. The aim of this procedure is to remove the primary organ that destroys the antibody-coated RBCs [Citation21]. Splenectomy was reported to be associated with a response rate of 60–90% [Citation19,Citation21,Citation22]. This procedure is indicated in patients with warm-type AIHA that have steroid dependency, resistance, or relapse [Citation20–22]. Nevertheless, no splenectomy was performed at our center in patients with primary warm-type AIHA during the past 10 years, and other studies also showed a very low rate of this procedure [Citation14–16,Citation23]. This low rate of splenectomy can be attributed to physicians’ concern regarding life-threatening complications, including overwhelming post-splenectomy infection and thrombosis [Citation24–27], and the fact that other non-invasive methods can also be used.
A 2015 meta-analysis of studies that used rituximab as a second-line or third-line treatment in primary warm-type AIHA showed a high overall response rate of approximately 80% [Citation28], with a superior side-effect profile to that observed when using immunosuppressive drugs [Citation6]. Other studies also suggested rituximab as a preferred second-line treatment [Citation29], and that combining it with steroids will yield a good response in warm-type AIHA [Citation30]. Importantly, rituximab was prescribed for only one patient in this study due to its high cost, and it is generally not covered by the Thai national health insurance scheme. Taken together, these findings suggest that the cost of rituximab should be covered by the national health insurance schemes in Thailand.
Regarding clinical and biochemical characteristics, the mean age at AIHA diagnosis was around 56 years, which is older than the median age reported from previous studies [Citation15,Citation31–33]. Those studies reported a high incidence of first diagnosis of AIHA among patients aged 15–40 in Asian population. Our finding suggests that Thai patients with primary warm-type AIHA who will be refractory to or relapsed while taking steroids tend to be older. Similar to our study in Thai patients, several studies reported a median age at onset of over 50 years among Caucasians [Citation4,Citation14,Citation16,Citation34,Citation35]. Our study also reported the median, minimum, and maximum of laboratory markers that represent indirect evidence of hemolysis (i.e. absolute reticulocyte count, lactate dehydrogenase, total bilirubin, and aspartate aminotransferase). The results of that analysis suggest that normal values of these markers are common and cannot be used to exclude active hemolysis.
Limitations
This study has some limitations. First, our retrospective review of a small number of patients made it impossible to draw a relationship between adverse events and treatment. For example, in other studies, thrombosis seems to correlate with AIHA disease severity [Citation36] or an abnormality of red blood cell membrane due to autoantibodies [Citation37] more than with azathioprine itself. Similarly, one of the two patients treated with danazol developed infection despite the fact that infection is not a documented side effect [Citation38,Citation39]. Second, our small sample size due to the relative rarity of the disease could not reflect the efficacy of treatments other than azathioprine and cyclophosphamide since one or the other of these two drugs was used to treat 90% of patients in this study.
Conclusion
Immunosuppressive drugs are the most common second-line treatment for primary warm-type AIHA in Thailand; however, they yield an unsustained response. Positive strong direct Coombs’ test was the only factor significantly associated with response outcome. Additional therapies are needed to reduce the relapse rate and prolong remission.
Acknowledgements
The authors gratefully acknowledge Professor Peter Hokland of the Department of Clinical Medicine, Department of Hematology, Aarhus University, Aarhus, Denmark, for his critical review of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Chattree Hantaweepant http://orcid.org/0000-0003-0996-3789
Weerapat Owattanapanich http://orcid.org/0000-0002-1262-2005
References
- Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69(4):258–271. http://www.ncbi.nlm.nih.gov/pubmed/11921020 . Accessed January 24, 2019. doi: 10.1002/ajh.10062
- Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4(6):607–618. doi:10.1586/ehm.11.60.
- Barcellini W. Immune hemolysis: diagnosis and treatment recommendations. Semin Hematol. 2015;52(4):304–312. doi:10.1053/j.seminhematol.2015.05.001.
- Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5–6):136–151. doi: 10.1007/s00508-008-0945-1
- Genty I, Michel M, Hermine O, et al. Characteristics of autoimmune hemolytic anemia in adults: retrospective analysis of 83 cases. La Rev Med Interne. 2002;23(11):901–909. http://www.ncbi.nlm.nih.gov/pubmed/12481390 . Accessed March 8, 2019. doi: 10.1016/S0248-8663(02)00688-4
- Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116(11):1831–1838. doi:10.1182/blood-2010-03-259325.
- Sokol RJ, Hewitt S, Stamps BK. Autoimmune hemolysis: mixed warm and cold antibody type. Acta Haematol. 1983;69(4):266–274. doi:10.1159/000206903.
- Shulman IA, Branch DR, Nelson JM, et al. Autoimmune hemolytic anemia with both cold and warm autoantibodies. JAMA. 1985;253(12):1746–1748. http://www.ncbi.nlm.nih.gov/pubmed/3974053 . Accessed September 10, 2019.9. doi: 10.1001/jama.1985.03350360072021
- Allgood JW, Chaplin H. Idiopathic acquired autoimmune hemolytic anemia. A review of forty-seven cases treated from 1955 through 1965. Am J Med. 1967;43(2):254–273. http://www.ncbi.nlm.nih.gov/pubmed/6034957 . Accessed January 24, 2019. doi: 10.1016/0002-9343(67)90168-4
- Murphy S, LoBuglio AF. Drug therapy of autoimmune hemolytic anemia. Semin Hematol. 1976;13(4):323–334. http://www.ncbi.nlm.nih.gov/pubmed/1006333. Accessed January 24, 2019.
- Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev. 2008;22(1):17–31. doi:10.1016/j.blre.2007.08.001.
- Crowther M, Chan YLT, Garbett IK, et al. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118(15):4036–4040. doi:10.1182/blood-2011-05-347708.
- Zanella A, Barcellini W. Treatment of autoimmune hemolytic anemias. Haematologica. 2014;99(10):1547–1554. doi:10.3324/haematol.2014.114561.
- Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–2936. doi:10.1182/blood-2014-06-583021.
- Rattarittamrong E, Eiamprapai P, Tantiworawit A, et al. Clinical characteristics and long-term outcomes of warm-type autoimmune hemolytic anemia. Hematology. 2016;21(6):368–374. doi:10.1080/10245332.2016.1138621.
- Roumier M, Loustau V, Guillaud C, et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: new insights based on a single-center experience with 60 patients. Am J Hematol. 2014;89(9):E150–E155. doi: 10.1002/ajh.23767
- Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. 2017. doi:10.1182/blood-2016-11
- Salama A. Treatment options for primary autoimmune hemolytic anemia: a short comprehensive review. Transfus Med Hemotherapy. 2015;42(5):294–301. doi:10.1159/000438731.
- Coon WW. Splenectomy in the treatment of hemolytic anemia. Arch Surg. 1985;120(5):625–628. http://www.ncbi.nlm.nih.gov/pubmed/3985801. Accessed February 21, 2019. doi: 10.1001/archsurg.1985.01390290099017
- Bonnet S, Guédon A, Ribeil J-A, et al. Indications and outcome of splenectomy in hematologic disease. J Visc Surg. 2017;154(6):421–429. doi:10.1016/j.jviscsurg.2017.06.011.
- Akpek G, McAneny D, Weintraub L. Comparative response to splenectomy in Coombs-positive autoimmune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61(2):98–102. http://www.ncbi.nlm.nih.gov/pubmed/10367787. Accessed February 21, 2019. doi: 10.1002/(SICI)1096-8652(199906)61:2<98::AID-AJH4>3.0.CO;2-G
- Patel NY, Chilsen AM, Mathiason MA, et al. Outcomes and complications after splenectomy for hematologic disorders. Am J Surg. 2012;204(6):1014–1020. doi:10.1016/j.amjsurg.2012.05.030.
- Barcellini W, Fattizzo B, Zaninoni A. Current and emerging treatment options for autoimmune hemolytic anemia. Expert Rev Clin Immunol. 2018;14(10):857–872. doi:10.1080/1744666X.2018.1521722.
- Krauth M-T, Lechner K, Neugebauer EAM, et al. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention–an unresolved issue. Haematologica. 2008;93(8):1227–1232. doi:10.3324/haematol.12682.
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–1981. doi:10.1161/CIRCULATIONAHA.110.015008.
- Bisharat N, Omari H, Lavi I, et al. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43(3):182–186. doi:10.1053/jinf.2001.0904.
- Davidson RN, Wall RA. Prevention and management of infections in patients without a spleen. Clin Microbiol Infect. 2001;7(12):657–660. http://www.ncbi.nlm.nih.gov/pubmed/11843905 . Accessed January 24, 2019. doi: 10.1046/j.1198-743x.2001.00355.x
- Reynaud Q, Durieu I, Dutertre M, et al. Efficacy and safety of rituximab in auto-immune hemolytic anemia: a meta-analysis of 21 studies. Autoimmun Rev. 2015;14(4):304–313. doi:10.1016/j.autrev.2014.11.014.
- Barcellini W. Current treatment strategies in autoimmune hemolytic disorders. Expert Rev Hematol. 2015;8(5):681–691. doi:10.1586/17474086.2015.1073105.
- Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163(3):393–399. doi:10.1111/bjh.12541.
- Kruatrachue M SS. Autoimmune hemolytic anemias in Thailand. Scan J Haematol. 1977;19(1):61–67. doi: 10.1111/j.1600-0609.1977.tb02719.x
- Dussadee K, Taka O, Thedsawad A, et al. Incidence and risk factors of relapses in idiopathic autoimmune hemolytic anemia. J Med Assoc Thail. 2010;93(Suppl. 1):165–170.
- Naithani R, Agrawal N, Mahapatra M, et al. Autoimmune hemolytic anemia in India: clinico-hematological spectrum of 79 cases. Hematology. 2006;11(1):73–76. doi:10.1080/10245330500345587.
- Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre. Br Med J (Clin Res Ed). 1981: 2023–2027. https://www.ncbi.nlm.nih.gov/pubmed/?term=sokol+RJ+autoimmune+haemolysis+865+cases . Accessed February 25, 2019. doi: 10.1136/bmj.282.6281.2023
- Michel M. Warm autoimmune hemolytic anemia: Advances in pathophysiology and treatment. Presse Med. 2014;43(4):e97–e104. doi:10.1016/j.lpm.2014.02.009.
- Lecouffe-Desprets M, Néel A, Graveleau J, et al. Venous thromboembolism related to warm autoimmune hemolytic anemia: a case-control study. Autoimmun Rev. 2015;14(11):1023–1028. doi:10.1016/j.autrev.2015.07.001.
- Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89(4):1121–1132. http://www.ncbi.nlm.nih.gov/pubmed/9028933 . Accessed January 24, 2019.
- Liu W, Gu X, Fu R, et al. The effect of danazol in primary immune thrombocytopenia: an analysis of a large cohort from a single center in China. Clin Appl Thromb Hemost. 2016;22(8):727–733. doi:10.1177/1076029615622002.
- Jaime-Pérez JC, Colunga-Pedraza PR, Gómez-Ramírez CD, et al. Danazol as first-line therapy for aplastic anemia. Ann Hematol. 2011;90(5):523–527. doi:10.1007/s00277-011-1163-x.
