ABSTRACT
Background: Cytomegalovirus (CMV) infection of the central nervous system (CNS) is a rare but life-threatening complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Cases presentation: Two patients with drug-resistant CMV encephalitis after allo-HSCT were successfully treated with donor CMV-specific cytotoxic T lymphocytes (CTLs). In the first case, a 27-year-old male who received haploidentical transplantation to treat T-cell acute lymphoblastic leukemia (T-ALL), developed CMV encephalitis during the time of the ganciclovir maintenance treatment. After intravenous foscarnet and donor CMV-specific CTLs, CMV-DNA of CSF became undetectable and the abnormal signs of brain magnetic resonance imaging (MRI) were limited. Another case, a 57-year-old female with acute myeloid leukemia (AML) who underwent haploidentical transplantation, also developed CMV encephalitis during the maintenance treatment of the ganciclovir. After administering donor CMV-specific CTLs intrathecally, the CMV load of the CSF decreased.
Conclusions: The intravenous/intratheca administration of donor CMV-specific CTLs may be a safe and effective treatment for CMV encephalitis, especially for patients who suffered from drug-resistant CMV infection.
Introduction
Before the availability of effective preventive therapies, cytomegalovirus (CMV) disease was a common and life-threatening complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). In recent years, the incidence of CMV disease after allo-HSCT has been significantly reduced by prophylaxis and/or preemptive treatment with ganciclovir (GCV) [Citation1,Citation2]. Digestive tract and lung are the most commonly involved organs of CMV reactivation after allo-HSCT, while the central nervous system (CNS) disease related with CMV reactivation is rare [Citation3–5]. However, the mortality rate of CMV encephalitis is high because of CMV-specific immunity impairment, antiviral drug resistance, and reduced efficacy of antiviral treatment due to the poor bioavailability of GCV, foscarnet (FCV) and cidofovir (CDV) in cerebrospinal fluid (CSF) [Citation6,Citation7]. Therefore, new therapeutic strategies for CNS disease caused by CMV reactivation are needed. In this article, two patients developed with CMV encephalitis after allo-HSCT were reported and successfully treated with donor CMV-specific cytotoxic T lymphocytes (CTLs).
Case presentation
Transplant approach
Both the patients received myeloablative conditioning regimen including Me-CCNU (250 mg/m2, day -10), cytarabine (4 g/m2/day, days -9 and -8), busulfan (4 mg/kg/day, days -7 to -5) and cyclophosphamide (1.8 g/m2/day, days -4 and -3). Bone marrow and peripheral blood grafts were infused on day 0. Cyclosporine at 3 mg/kg/day was given by continuous infusion over 24 h from day -10, with a target blood concentration ranging from 200 to 300 ng/ml. Short-term methotrexate was given at 15 mg/kg/day on day +1 and 10 mg/kg/day on days +3, +6 and +11. Oral intake of mycophenolate mofetil was given at 1.0 g twice daily from day -10 to day +30, and then gradually tapered off until day +60. Rabbit antithymocyte globulin (r-ATG, Genzyme, Cambridge, MA, USA) was administrated in vivo at 2.5 mg/kg on days -5 and -2. These drugs were given as graft-versus-host disease (GVHD) prophylaxis.
Virologic monitoring and treatment
All patients and donors accepted CMV-DNA quantification test before transplantation. Acyclovir was given as herpes prophylaxis for patients after allo-HSCT. All patients received frequent virologic monitoring, and preemptive treatment strategies were administered if necessary for most common viral infections, such as CMV, Epstein–Barr virus (EBV), etc. [Citation8–10].
In more detail, for patients without CMV reactivation, real-time PCR for CMV-DNA quantification with peripheral blood was performed once a week from day 0 to day +90, once every two weeks from day +91 to day +180, and then once every four weeks from day +181 to day +360. While for patients who suffered from CMV reactivation, CMV-DNA quantification was performed twice a week. Treatment with GCV was initiated when the viral load >1 × 104 copies/ml [Citation11]. UL97 and UL54 genes of CMV were sequenced when GCV- or FCV-resistant CMV strains emerged [Citation12].
Donor CMV-specific CTLs prepare
Human cytomegalovirus (HCMV) seropositive donors have memory virus-specific T cells. Peripheral blood mononuclear cells (PBMCs) of the donor are isolated by lymphoprep fluid. 1 × 109 PBMCs with a cell density of 1 × 107/ml in serum-free media (Gibco Company, USA) were used, with a mixture of pp65 and immediate early (IE)-1 peptides (Miltenyi Biotec) in 37°C incubator to stimulate 6 h. PBMCs were coated with IFN-γ capture matrix and cultured for 45 min at 37°C. After stimulation, HCMV-specific T cells secrete IFN-γ, and then these cells were aseptically sorted by IFN-γ cytokine enrichment reagent.
Patient No. 1
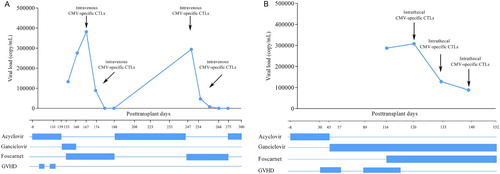
A 27-year-old man with T-ALL in first complete hematological remission (CR1) underwent HSCT from his father (5/10 HLA compatible, HLA-A, -B and -DRB1 mismatched) on October 17th, 2014. Both recipient and donor were CMV-IgG seropositive and CMV-IgM seronegative. Engraftment was achieved on day +14, with a full donor chimerism. Acute GVHD (aGVHD) grade 2 (gastrointestinal involved) was shown on day +39, and then successfully treated with methylprednisolone (2 mg/kg/day) and tacrolimus (0.03 mg/kg/day). On day +95, grade 3 aGVHD of gastrointestinal was recurrent again, and then was resolved by methylprednisolone (2 mg/kg/day), tacrolimus (0.03 mg/kg/day) and CD25 monoclonal antibody (20 mg/week × 4 cycles). The immunosuppressants were gradually tapered off and finally discontinued on day +180 with no signs of GVHD. On day +139, the patient developed headache and fever, with a negative EBV-DNA and CMV-DNA test in the peripheral blood. We replaced acyclovir with GCV, and combined imipenem-cilastin sodium and voriconazole to treat infection, but in vain. On day +153, the patient’s headache became dreadful, accompanied by drowsy, and brain magnetic resonance imaging (MRI) showed multiple abnormal long T1 and T2 signals at the bilateral lateral ventricle, which highly suggested infection. CSF results showed 282 cells/ml (neutrophils 64% and lymphocytes 26%), increased protein levels (1.14 g/L, normal range 0.15–0.45 g/L) and decreased glucose levels (2.35 mmol/L, normal range 2.5–4.5 mmol/L). No malignant cells were found by the cytological and flow-cytometry analysis in the CSF. However, CMV-DNA quantification of CSF was 1.33 × 105 copies/ml, while PCR results for EBV-DNA, herpes simplex virus (HSV) -1/2, human herpesvirus (HHV) -6/8, varicella zoster virus (VZV), BK virus (BKV) and enteroviruses were negative. CSF cultures and serologic tests for bacteria, fungi, mycobacteria and parasite were also negative. Quantification PCR of CMV-DNA and EBV-DNA in the peripheral blood was persistent negative. We added FCV (90 mg/kg twice daily) to GCV (5 mg/kg twice daily). However, in the following two weeks, the patient’s headache and drowsy did not relieve. And then the GCV was withdrawn due to the sequence analysis of UL54 and UL97 mutation genes in CMV-DNA from CSF samples. On day +167, after obtaining consent from the patient, donor CMV-specific CTLs (1 × 105 CMV-CTLs/kg) were intravenously administered. On day +174, virus load in CSF decreased (8.9 × 104 copies/ml), then we administrated donor CMV-specific CTLs once again, with the same CTL doses. On day +181, CMV-DNA in CSF became undetectable and the abnormal signs of brain MRI were limited on day +203. After two months, on day+247, the patient repeated fever, headache and consciousness impairment; and CMV-DNA in CSF was recurrent (2.94 × 105 copies/ml). Treatment with FCV was initiated, and donor CMV-specific CTLs were infused on days +247 and +254, then clinical symptoms and CMV-DNA in CSF disappeared on day +268. In the whole course, no aGVHD related to CMV-specific CTLs was observed. The patient is currently alive without any signs of infection or GVHD. The virologic follow-up is summarized in (A).
Patient No. 2
A 57-year-old female with AML underwent allo-HSCT in CR1 from her daughter (6/10 HLA compatible, HLA-B and -DRB1 mismatched) on 29 March 29 2018. Both recipient and donor were CMV-IgG seropositive and CMV-IgM seronegative. Engraftment was achieved on day +14, with a full donor chimerism. Acute GVHD grade 3 (both gastrointestinal and skin involved) was presented on day +30, and then was successfully treated with methylprednisolone, tacrolimus (0.03 mg/kg/day) and CD25-monoclonal antibody. The immunosuppressants were gradually tapered off and only tacrolimus was reserved finally. On day +43, CMV-DNA level of peripheral blood was 4.27 × 104 copies/ml, and preemptive therapy with intravenous GCV (5 mg/kg twice daily) was started, and CMV-DNA load of peripheral blood became undetectable on day +85. However, surveillance testing revealed recurrent CMV viremia on day+116 when GCV was still used. At the same time, the patient presented progressive consciousness impairment, and the brain MRI scan of which showed multiple abnormal long T1 and T2 signs in the cerebellar hemisphere, centrum semiovale and corona radiate. CSF results showed 310 cells/ml (neutrophils 79% and lymphocytes 11%), increased protein level (0.82 g/L) and increased glucose level (4.97 mmol/L), but no malignant cells were found by cytological and flow-cytometry analysis. CMV-DNA quantification of CSF was 2.83 × 105 copies/ml, while PCR results for EBV-DNA, HSV-1/2, HHV-6/8, VZV, BKV and enteroviruses were negative. CSF cultures and serologic tests for bacteria, fungi, mycobacteria and parasite were also negative. Meanwhile, the patient’s condition became complicated with grade 3 GVHD of gastrointestinal. Sequence analysis of CMV-DNA from CSF samples showed mutation in UL54 and UL97 genes, which is known to confer on drug-resistance. Therefore, we added FCV (90 mg/kg twice daily). On day +126, virus load of CMV in CSF increased (3.05 × 105 copies/ml), after obtaining the consent from the patient, donor CMV-specific CTLs (1 × 105 CMV-CTLs/kg) were intrathecally administered, in order to avoid aggravating GVHD. No adverse effects related to the intrathecal administration of CMV-specific CTLs, such as neurological symptoms (fever, headache, seizures, somnolence and others) and allergic reactions, were observed. On day +133, viral load of CMV (1.25 × 105 copies/ml) was detected in the CSF, and the donor CMV-specific CTLs intrathecal administration was performed again. During the process, the CMV load in CSF of the patient decreased progressively (8.5 × 104 copies/ml on day +140). Therefore, we performed the third intrathecal administration of the donor CMV-specific CTLs on day +140. However, unfortunately the patient died of severe septic shock without progressed GVHD or CMV encephalitis on day +152. The virologic follow-up is summarized in (B).
Discussion
Lung and gastrointestinal are the mainly involved targeting organs of CMV reactivation after allo-HSCT, and the CMV diseases of which were more commonly characterized by interstitial pneumonia and gastrointestinal disease. However, CMV encephalitis has been seldom reported [Citation3], which may be due to the difficulties of diagnosis and the controversies about the pathogenesis of CMV encephalitis.
CMV encephalitis is a relatively late complication after allo-HSCT. In a review of 11 patients suffering from CMV encephalitis after allo-HSCT, Reddy et al. [Citation4] reported that the median time of onset of CMV encephalitis was +210 (166–285) days. There was also another study about the onset of CMV encephalitis, and the median time of which was+105 (95–114) days [Citation3]. Our two patients both occurred over 4 months after transplantation. The reason of these different times may depend on the differences of host’s immunity condition. Computed tomography (CT)- and MRI-scan of the brain usually show changes of encephalitis, while MRI has greater diagnostic values than CT [Citation3]. However, to prove CMV encephalitis not only require CNS symptoms but also require the detection of CMV-DNA in CSF or/and brain tissue by quantitative PCR.
Previous studies indicated several factors that appeared to contribute to the development of CMV encephalitis, such as T-cell-depleted stem-cell grafts or treatment with T-cell-depleting agents or sub-optimal antiviral drug penetration, history of antiviral treatment with multiple courses, or immunosuppressive state [Citation13–15]. Both of our two cases underwent allo-HSCT with T-cell depletion in vivo by ATG and long-term antiviral therapy, which might contribute to the development of CMV encephalitis. Interestingly, for case 1, CMV-DNA was consecutively negative in peripheral blood while positive in CSF. It seems that CMV viral load in peripheral blood may not adequately reflect viral replication in sequestered sites, such as CNS, which has been reported in other cases [Citation16]. So, the most crucial questions are not for surveillances in the peripheral blood, but for when and how to monitor CMV-DNA in the CSF.
There are four common antiviral agents (GCV, acyclovir, FCV, CDV) approved for CMV infection. Nevertheless, relatively low penetration into the CNS (corresponding to 24–67% of plasma levels) and drug resistance significantly reduce the effectiveness of antiviral drugs in CMV encephalitis. Previous researches have showed that the mortality of CMV encephalitis after allo-HSCT was very high. Sequencing of CMV UL97 and/or UL54 mutation indicated drug-resistant CMV strains in CSF, blood or other tissues [Citation4]. Importantly, the reduction of immunosuppression to the greatest extent possible is crucial to the treatment of CMV-diseases; however, it must be balanced against the risk of active GVHD. If patients both developed GVHD and CMV-diseases after allo-HSCT, it meant the reduction of Immunosuppression is impossible.
On this occasion, CMV-specific CTLs may alter the course of the disease. It is an adoptive immunotherapy, which could quickly reconstitute antiviral immunity and especially kill CMV. Studies of immune recovery after allo-HSCT showed a temporal delay in the recovery of CMV-specific T-cell responses and have identified a decisive role for the recovery of CMV-specific CTLs responses in preventing the development of CMV disease [Citation17,Citation18]. Several previous studies have also shown the efficacy of infusing CMV-specific CTLs for the treatment and prevention of CMV reactivation after HSCT [Citation19–24]. Riddell et al. [Citation22] initially reported the safety and efficacy of infusing stem cell donor-derived CTLs clones, but this approach was not applied on a larger scale because of the technical challenges associated with cell cloning. Bao et al. [Citation23] indicated that the infusion of CTLs stimulated over 1–2 weeks with overlapping CMV peptides can result in virus-specific immune reconstitution in HSCT recipients, and five of the seven subjects who with refractory CMV infections had new onset CMV-specific CTL activity after infusion. Otherwise, Einsele et al. [Citation24] generated CMV CTLs by repetitive stimulation with CMV lysate, and 5/7 patients had sustained anti-CMV immune responses. As seen in our case 1, two low doses of intravenous CMV-specific CTLs led to the clearance of CMV in CSF, and still effective when CMV encephalitis relapse. No adverse effects related to the special CTLs, such as GVHD, were observed during the procedure.
Intravenous administration of CMV-specific CTLs can directly kill CMV, but might probably induce or aggravate GVHD [Citation25]. In our study, when case 2 developed CMV encephalitis, he both suffered from grade 3 aGVHD of gastrointestinal after allo-HSCT. Therefore, to avoid aggravation of aGVHD, we chose the intrathecal administration of CMV-specific CTLs instead to treat CMV encephalitis. The results showed that the intrathecal administration of CMV-specific CTLs led to the decrease of viral load in CSF while no aggravate GVHD or other local adverse effects were presented. Therefore, intrathecal administration of CMV-specific CTLs may be worth trying, especially for patients both with aGVHD. Nevertheless, it is worth noting that some studies have reported that a significant reduction of CMV-specific CTLs occurred with immunosuppressive therapies [Citation26,Citation27]. Another study has also shown that the power of cytokine production of the CMV-specific CD8+ T cells decreased in patients with immunosuppressive therapies [Citation28]. So, the combined treatment with immunosuppressive therapies may influence the effectiveness of CMV-specific CTLs, which may need to be proven in further researches. To our knowledge, ours is the first reported case where CMV encephalitis was treated with the combination therapy of intrathecal CMV-specific CTLs and antiviral drugs. However, the safety and efficacy of intrathecal CMV-specific CTLs need to be confirmed by the further studies.
Conclusions
CMV encephalitis after allo-HSCT is a rare but life-threatening complication. In cases of CNS involvement, antiviral drugs may be not enough for adequate clinical response, especially for patients suffering from drug-resistant CMV infection. Alternative methods need to be exploited besides antiviral medicines. CMV-specific CTLs may be an optional therapy, although the safety and efficacy require further study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9(9):543–558. doi: 10.1016/S1083-8791(03)00287-8
- Boeckh M, Gooley TA, Myerson D, et al. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063–4071. doi: 10.1182/blood.V88.10.4063.bloodjournal88104063
- Schmidt-Hieber M, Schwender J, Heinz WJ, et al. Viral encephalitis after allogeneic stem cell transplantation: a rare complication with distinct characteristics of different causative agents. Haematologica. 2011;96(1):142–149. doi: 10.3324/haematol.2010.029876
- Reddy SM, Winston DJ, Territo MC, et al. CMV central nervous system disease in stem-cell transplant recipients: an increasing complication of drug-resistant CMV infection and protracted immunodeficiency. Bone Marrow Transplant. 2010;45(6):979–984. doi: 10.1038/bmt.2010.35
- Maschke M, Kastrup O, Diener H C. CNS manifestations of cytomegalovirus infections: diagnosis and treatment. CNS Drugs. 2002;16(5):303–315. doi: 10.2165/00023210-200216050-00003
- Mori T, Kato J. Cytomegalovirus infection/disease after hematopoietic stem cell transplantation. Int J Hematol. 2010;91(4):588–595. doi: 10.1007/s12185-010-0569-x
- Wolf DG, Lurain NS, Zuckerman T, et al. Emergence of late cytomegalovirus central nervous system disease in hematopoietic stem cell transplant recipients. Blood. 2003;101(2):463–465. doi: 10.1182/blood-2002-07-1982
- Baldanti F, Lilleri D, Gerna G. Human cytomegalovirus load measurement and its applications for pre-emptive therapy in patients undergoing hematopoietic stem cell transplantation. Hematol Oncol. 2008;26(3):123–130. doi: 10.1002/hon.856
- Baldanti F, Gatti M, Furione M, et al. Kinetics of Epstein-Barr virus DNA load in different blood compartments of pediatric recipients of T-cell-depleted HLA-haploidentical stem cell transplantation. J Clin Microbiol. 2008;46(11):3672–3677. doi: 10.1128/JCM.00913-08
- Comoli P, Basso S, Labirio M, et al. T cell therapy of Epstein-Barr virus and adenovirus infections after hemopoietic stem cell transplant. Blood Cells Mol Dis. 2008;40(1):68–70. doi: 10.1016/j.bcmd.2007.06.020
- Lilleri D, Gerna G, Furione M, et al. Use of a DNAemia cut-off for monitoring human cytomegalovirus infection reduces the number of preemptively treated children and young adults receiving hematopoietic stem-cell transplantation compared with qualitative pp65 antigenemia. Blood. 2007;110(7):2757–2760. doi: 10.1182/blood-2007-03-080820
- Chou S, Ercolani RJ, Sahoo MK, et al. Improved detection of emerging drug-resistant mutant cytomegalovirus subpopulations by deep sequencing. Antimicrob Agents Chemother. 2014;58(8):4697–4702. doi: 10.1128/AAC.03214-14
- Jain NA, Lu K, Ito S, et al. The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation-implications for preventative treatment approaches. Cytotherapy. 2014;16(7):927–933. doi: 10.1016/j.jcyt.2014.02.010
- Fletcher C, Sawchuk R, Chinnock B, et al. Human pharmacokinetics of the antiviral drug DHPG. Clin Pharmacol Ther. 1986;40(3):281–286. doi: 10.1038/clpt.1986.177
- Abu-Khader A, Krause S. Rapid monitoring of immune reconstitution after allogeneic stem cell transplantation – a comparison of different assays for the detection of cytomegalovirus-specific T cells. Eur J Haematol. 2013;91(6):534–545. doi: 10.1111/ejh.12187
- Sarva H, Graber J, Remanan R, et al. CMV encephalitis in BMT recipients. Bone Marrow Transplant. 2012;47(2):318–320. doi: 10.1038/bmt.2011.80
- Quinnan GJ, Kirmani N, Rook AH, et al. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982;307(1):7–13. doi: 10.1056/NEJM198207013070102
- Reusser P, Riddell SR, Meyers JD, et al. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78(5):1373–1380. doi: 10.1182/blood.V78.5.1373.1373
- Comoli P, Maccario R, Locatelli F, et al. Adoptive transfer of herpesvirusspecific cytotoxic t lymphocytes in transplant recipients. Herpes. 2000;7(1):9–12.
- Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475
- Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362(9393):1375–1377. doi: 10.1016/S0140-6736(03)14634-X
- Riddell SR, Watanabe KS, Goodrich JM, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912
- Bao L, Cowan M J, Dunham K, et al. Adoptive immunotherapy with CMV-specific cytotoxic T lymphocytes for stem cell transplant patients with refractory CMV infections. J Immunother. 2012;35(3):293–298. doi: 10.1097/CJI.0b013e31824300a2
- Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–3922. doi: 10.1182/blood.V99.11.3916
- Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(6):707–714. doi: 10.1016/j.bbmt.2007.02.004
- Aubert G, Hassan-Walker AF, Madrigal JA, et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis. 2001;184(8):955–963. doi: 10.1086/323354
- Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97(5):1232–1240. doi: 10.1182/blood.V97.5.1232
- Engstrand M, Lidehall AK, Totterman TH, et al. Cellular responses to cytomegalovirus in immunosuppressed patients: circulating CD8+ T cells recognizing CMVpp65 are present but display functional impairment. Clin Exp Immunol. 2003;132(1):96–104. doi: 10.1046/j.1365-2249.2003.02098.x