ABSTRACT
Background: Antinuclear antibodies (ANAs) can be detected in about 30% of patients with primary immune thrombocytopenia (ITP), yet their relationship with treatment response to rituximab remains elusive.
Methods: we retrospectively reviewed the clinical records of hospitalized adult ITP patients who were treated with rituximab from three medical centers across China. Rituximab was given intravenously at 100 mg weekly for 4 weeks, or at a single dose of 375 mg/m2. All included patients had their ANAs tested before rituximab treatment.
Results: A total of 287 patients fulfilled the inclusion criteria and were eligible for analysis. ANAs were positive in 98 (34.1%) of the included patients. The incidence of overall response and complete response (CR) in ANA-positive patients was significantly higher than that in ANA-negative patients (overall response: 76.5% vs. 55.0%, P < 0.001; CR: 46.9% vs. 29.1%, P = 0.003). However, sustained response (SR) rates in ANA-positive patients at 6, 12 and 24 months were all lower compared with ANA-negative patients (all P < 0.05). The overall duration of response (DOR) estimated by Kaplan–Meier analysis in ANA-negative patients was greater than that in ANA-positive patients (P < 0.001).
Conclusion: ITP patients with positive ANA test were likely to achieve a better initial response to rituximab treatment, while their long-term outcome was unfavorable. Therefore, ANA test could be useful for predicting rituximab response in ITP.
Background
Primary immune thrombocytopenia (ITP) is an acquired organ-specific autoimmune disorder characterized by transient or persistent decrease of the peripheral blood platelet count to less than 100 × 109/L in the absence of conditions known to cause thrombocytopenia [Citation1]. The clinical manifestations of ITP vary from asymptomatic to localized hemorrhage in skin or mucous membranes, even severe bleeding events such as gastrointestinal hemorrhage, or intracranial hemorrhage. The main pathogenesis of ITP is the loss of immune tolerance to platelet auto-antigens, which results in increased platelet destruction and impaired thrombopoiesis by autoantibodies and cytotoxic T lymphocytes (CTLs) [Citation2,Citation3]. The diagnosis of ITP is still based on an exclusion criterium. Other causes of thrombocytopenia, such as systemic lupus erythematosus (SLE), myelodysplastic syndrome (MDS), lymphoproliferative disorders, and drug-related thrombocytopenia must be excluded before ITP diagnosis [Citation1].
Serologic tests for autoantibodies, including antinuclear antibodies (ANAs) and antibodies to specific nuclear antigens such as double-stranded DNA (dsDNA), play important roles in the diagnosis of systemic rheumatic diseases. ANAs are the most commonly used screening markers for SLE, systemic sclerosis, and other systemic autoimmune disorders [Citation4,Citation5]. However, the results of ANAs can be misleading. A positive ANA test is not completely sensitive or specific, as it can be detected quite commonly in patients with nonrheumatic diseases and even among healthy population [Citation6,Citation7]. Approximately 25–39% of ITP patients have detectable ANAs [Citation8,Citation9], while its clinical significance is not well documented. It has been reported that the positivity of ANAs in ITP patients was associated with a more chronic course, and a higher risk for developing systemic autoimmune disorders [Citation9,Citation10].
Corticosteroids and intravenous immunoglobulin (IVIG) remain the first-line treatments for ITP. Approximately one-third of patients do not respond to corticosteroids. Besides, a certain proportion of responders will relapse after corticosteroid discontinuation. Second-line treatments for corticosteroid-resistant or relapsed patients include thrombopoietic agents, immunosuppressive agents and splenectomy. Rituximab is a chimeric-monoclonal antibody against CD20. It has been frequently used for the management of ITP and recommended as a second-line treatment strategy. Rituximab can induce a long-term response and a splenectomy-sparing effect in nearly 20–40% of ITP patients who previously failed at least a full course of corticosteroid therapy [Citation11]. Despite the widespread use of rituximab in ITP, indicators for predicting the response to rituximab are still lacking. It has been reported that the absence of anti-glycoprotein autoantibodies was associated with a poor response to rituximab [Citation12], while our previous clinical trial did not observe such relationship [Citation13]. In the present study, we performed a retrospective analysis of adult ITP patients who were treated with rituximab from three medical centers in China, and found that response rate was higher, but duration of response (DOR) was shorter in ANA-positive patients.
Materials and methods
Patients
In this consecutive cohort study, we retrospectively reviewed the clinical records of adult ITP patients hospitalized between January 2012 and December 2018 in the hematology units of three different hospitals across China (Qilu Hospital, Shandong University; Yantai Yuhuangding Hospital; and Taian Central Hospital). All included patients met the International Working Group (IWG) diagnostic criteria for primary ITP [Citation1] and had their ANAs tested before rituximab treatment. Thrombocytopenic cases that were due to connective tissue diseases, such as systemic lupus erythematosus (SLE) or primary Sjögren syndrome (pSS) were excluded. Patients who developed SLE or other connective tissue diseases during follow-up were also excluded. Rituximab was given intravenously at 100 mg weekly for 4 weeks, or a single dose of 375 mg/m2. We excluded patients with a history of any ITP-specific treatment administered concomitantly with rituximab. Patients’ clinical and biological data regarding age, gender, disease duration, bleeding events, presence of other pathological conditions, previous treatments, and blood test results were collected. Bleeding severity was assessed according to ITP-specific assessment system [Citation14] based on the medical records of patient history and physical examination. We excluded age from the original scale and only recorded the bleeding manifestation scale, which was calculated by adding the points relevant to different clinical bleeding symptoms. The ethics committee waived the need for informed consent for this retrospective study because of the absence of impact on the management of patients. Three experts independently reviewed the medical records of each patients and extracted the study features. Discrepancies were resolved by discussion until consensus was reached.
Laboratory tests
All the laboratory evaluations before rituximab treatment were performed according to the standard methods at each participating site. Laboratory tests included routine blood count, blood film and human immunodeficiency virus (HIV), hepatitis C and B virus evaluation, serum IgG level. Megakaryocytes were counted on bone marrow smear of 4.5 cm2 area (1.5 × 3.0 cm2) at low magnifications if bone marrow examination was performed. ANAs were determined by the immune fluorescent technique on Hep-2 cell substrate. Positive results were defined as a titer pf 1:80 or more.
Treatment and response
Rituximab was given intravenously either at 100 mg weekly for 4 weeks or a single dose of 375 mg/m2. Response was evaluated according to the recommended definitions of IWG [Citation1] within 3 months of the first rituximab injection. The criteria for response were listed as follows: (1) complete response (CR): platelet count ≥100 × 109/L and absence of bleeding, (2) response (R): platelet count ≥30 × 109/L and absence of bleeding, and (3) no response (NR): platelet count < 30 × 109/L or < two-fold increase of the baseline platelet count or bleeding. Relapse was defined as a platelet count decreased to < 30 × 109/L and/or the presence of bleeding in responders. Time to response (TTR) was defined as the time from the first rituximab injection to response. The time from response to relapse was regarded as DOR which was collected by going through the follow-up data of responders.
Statistical analysis
Data were expressed as means ± standard deviation (SD) or median (range) for continuous values and counts (percentages) for categorical values. We compared age, disease duration, baseline platelet counts, serum IgG levels, megakaryocyte counts, TTR, and DOR between patients with or without ANAs using Student t-test or Mann–Whitney U test, as appropriate. Differences in rates of overall response, and CR between patients with and without ANAs were compared using Chi-Square test. Kaplan–Meier method and log-rank test was performed to evaluate the difference in DOR between ANA-positive and ANA-negative responders. Multivariable logistic regression analysis was used to quantify the effect of variables on rituximab response. A two-sided P value < 0.05 was considered statistically significantly. Data management and statistical analyses were performed using SPSS, version 23.0.
Results
Patients’ characteristics
A total of 287 rituximab-treated ITP patients with available ANA test results were included in this study. The median (range) age of patients was 53 (16–86) years. There were 184 (64.1%) female patients and 103 (35.9%) male patients. Aside from corticosteroids, intravenous immunoglobulin (IVIG) and recombinant human thrombopoietin (rhTPO) were the most commonly used medicines before rituximab infusion. The median duration of ITP from diagnosis to rituximab use was 6 (3–460) months. Median platelet count was 12 (0–33) × 109/L and the median bleeding score was 0 (0–5). The median number of the megakaryocyte was 101 (16–389). Among all patients, median serum IgG levels was 11.5 (6.6–19.7) g/L. None of the rituximab-treated patients showed seropositive detection of HIV, or virus copy of hepatitis C or B.
Association between ANAs and presenting characteristics
ANAs were positive in 98 (34.1%) of the 287 ITP patients. There was no difference in age, gender, disease duration, baseline platelet count, bleeding score, serum immunoglobulin (Ig) G level or bone marrow megakaryocyte count between ANA-positive patients and ANA-negative patients (all P > 0.05; ).
Table 1. Patient demographics and baseline characteristics.
ANAs and response to rituximab
Of the 287 eligible patients, 179 (62.4%) responded initially to rituximab treatment within 3 months, including 101 cases with CR. The median (range) TTR was 19 (10–45) days from the start of therapy. Of the 179 responders, 103 (35.9%) maintained the response after 6 months. Response was kept in 72 (25.1%) patients for 12 months and 42 (14.6%) patients for 2 years, and the median DOR was 201 (37–2920) days.
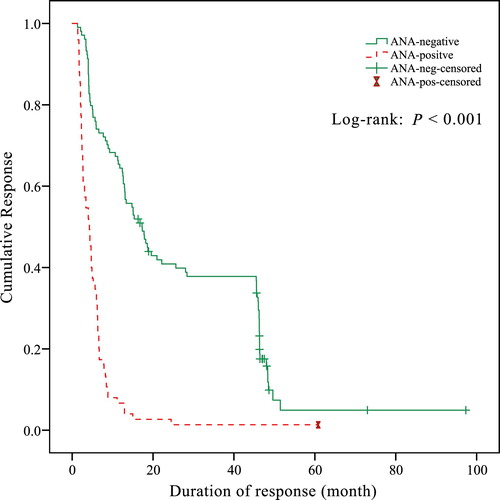
As shown in , the incidence of overall response in ANA-positive patients was significantly higher than that in ANA-negative patients (76.5% vs. 55.0%, P < 0.001). Additionally, the incidence of CR also showed statistical difference between ANA-positive patients and ANA-negative patients (46.9% vs. 29.1%, P = 0.003). There was no statistical difference in TTR between ANA-positive responders and ANA-negative responders (28 days vs. 30 days, P = 0.087). However, the DOR in ANA-positive patients was significantly shorter than that in ANA-negative patients (128 days vs. 503 days, P < 0.001).
Table 2. Responses to rituximab in ANA-positive and ANA-negative patients.
Multivariate logistic regression analysis revealed that age, gender, disease duration, baseline platelet count, serum IgG level, bone marrow megakaryocyte count, and rituximab dosage regimen were not associated with patients’ response. ANAs remained the predictive factor for better response to rituximab treatment. The odd ratio (OR) of response for ANA-positive patients to ANA-negative patients was 2.529 (P = 0.001; 95% CI: 1.447–4.420; ).
Table 3. The effect of baseline characteristics on rituximab response by multivariable logistic regression analysis.
Kaplan–Meier analysis revealed that DOR in ANA-positive patients was significantly shorter compared with ANA-negative patients (P < 0.001; ).
Discussion
ITP is an autoimmune disorder caused by accelerated platelet destruction and impaired platelet production. The diagnosis of ITP relies on the exclusion of other causes of thrombocytopenia, as there is no ‘gold standard’ diagnostic test to confirm ITP. Secondary ITP refers to all the other forms of immune-mediated thrombocytopenia except primary ITP. The distinction between primary and secondary ITP is clinically relevant because of their different pathobiology. Thrombocytopenia secondary to connective tissue diseases, especially SLE, are the most commonly encountered forms of secondary ITP [Citation15]. ANAs are the best screening markers for autoimmune disorders [Citation16,Citation17]. However, as pointed out by the international consensus report guidelines, the coexistence of ANAs on their own in the absence of distinctive clinical manifestations suggestive of SLE does not qualify the diagnosis of secondary ITP [Citation1]. In the present study, primary ITP patients were selected according to strict inclusion criteria, whereas they still had the possibility of developing secondary ITP. Our results showed that 34.1% of patients had detectable ANAs, which were in accordance with previously published data [Citation8,Citation9].
The terms ‘ANAs’ have been outdated and come to encompass antibodies against various cellular compartments such as nuclear constituents, components of the nuclear envelop, and mitotic spindle apparatus [Citation18]. As platelets are anucleate cytoplasts, there is still no evidence that ANAs can cross-react with platelet antigens and lead to antibody-mediated platelet destruction. Furthermore, no association was observed between ANAs and any of the patients’ baseline characteristics. Although bleeding symptoms seem to be more obvious in ANA-positive patients, no statistical significance was obtained. It has been reported that ANA positivity could act as an indicator in terms of chronicity for childhood ITP [Citation19]. By contrast, no correlation was found between ANAs and patients’ disease duration in our study. The discrepancy maybe partly due to the fact that we only recorded the time period from disease onset to rituximab use. Moreover, the difference in patients’ age and previous treatment history might also contribute to the discrepancy about the relationship between ANA positivity and disease chronicity. More importantly, this might also be part of the difference between adult ITP and pediatric ITP.
Corticosteroids are the standard first-line treatment for ITP with relatively high response rates. Abbasi et al. reported that ANA-negative ITP patients were more responsive to corticosteroid therapy compared to ANA-positive patients [Citation20]. For patients who failed the first-line treatment, rituximab and thrombopoietic agents could be used. Rituximab is a monoclonal anti-CD20 antibody which can induce remission in approximately 60% of ITP patients at the recommended dosage of 375 mg/m2 weekly for 4 weeks [Citation21,Citation22]. Due to the potential toxicity, several studies assessed lower dosing schedules. Results showed that rituximab at a dosage of 100 mg weekly for 4 weeks [Citation11,Citation13,Citation23], or a single dose of 375 mg/m2 [Citation24], possessed similar therapeutic efficacy but longer TTR compared to the standard dose. Rituximab is a relatively high-cost treatment, and excluding rituximab non-responders from this treatment could decrease unnecessary rituximab consumption. Two independent studies reported that a better response to rituximab was associated with the presence of anti-glycoprotein (GP) autoantibodies [Citation12,Citation25]. So far, the influence of ANAs on treatment response to rituximab in ITP remains unelucidated. Brah et al. found an increase in the response rate in ANA-positive ITP patients compared with ANA-negative cases, but statistical significance was not achieved [Citation26]. By contrast, our results demonstrated that rituximab induced significantly higher initial overall response rate and CR rate in ANA-positive patients than in ANA-negative patients. It has been reported that median TTR of rituximab in ITP was about 4–5 weeks, while a small part of patients might respond slowly to rituximab treatment, even until the end of month 3 [Citation11,Citation13,Citation27,Citation28]. Consistently, median TTR of all patients enrolled in our study was about 4 weeks. There was no statistical difference in TTR between ANA-positive patients and ANA-negative patients. Nevertheless, it was notable that the DOR was significantly higher for ANA-negative responders compared to those with a positive ANA test.
By presenting antigens to CD4+ T cells and secreting inflammatory cytokines capable of activating macrophages, B cells play important roles in the pathogenesis of ITP [Citation29]. Moreover, abnormally activated B cells can develop into plasma cells which further exacerbate platelet destruction by secreting anti-GP autoantibodies in ITP [Citation30–32]. Rituximab exerts its therapeutic effect in ITP mainly through depletion of pre-plasma B cells, thus decreasing autoantibody production [Citation32]. In addition, rituximab could correct the number and dysfunction of the T-cell compartment in ITP patients [Citation33–35]. The reason why ITP patients with ANAs could achieve a higher overall response rate remains unknown. We speculated that the presence of ANAs might be an indicator for more severe abnormality of humoral immunity, which could be alleviated by rituximab treatment. This might also account for the poorer long-term outcomes in ANA-positive patients. However, as the components of ANAs are complex, their effect on platelet destruction and thrombopoiesis still awaits further investigation. Furthermore, it is of great significance for the study of the pathogenesis and treatment of ITP to explore the therapy mechanism of different ITP responders.
Conclusions
In conclusion, our study found that ITP patients with positive ANA test were likely to achieve a better response after rituximab treatment, but their DOR was poorer compared with ANA-negative patients; therefore, ANA screening could be a useful test for predicting rituximab response in ITP.
Ethics approval and consent to participate
The ethics committee waived the need for informed consent for this retrospective study because of the absence of impact on the management of patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–186. doi: 10.1182/blood-2009-06-225565
- Audia S, Mahevas M, Samson M, et al. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620–632. doi: 10.1016/j.autrev.2017.04.012
- Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. doi: 10.3390/jcm6020016
- Stochmal A, Czuwara J, Trojanowska M. Antinuclear antibodies in systemic sclerosis: an update. Clin Rev Allergy Immunol. 2020;58(1):40–51. doi: 10.1007/s12016-018-8718-8
- Tedeschi SK, Johnson SR, Boumpas D, et al. Developing and refining new candidate criteria for systemic lupus erythematosus classification: an international collaboration. Arthritis Care Res (Hoboken). 2018;70(4):571–581. doi: 10.1002/acr.23317
- Pisetsky DS. Antinuclear antibody testing – misunderstood or misbegotten? Nat Rev Rheumatol. 2017;13(8):495–502. doi: 10.1038/nrrheum.2017.74
- Prapinjumrune C, Prucktrakul C, Sooktonglarng T, et al. Serum antinuclear antibody in adult Thais. Gerodontology. 2017;34(1):86–89. doi: 10.1111/ger.12233
- Grimaldi-Bensouda L, Nordon C, Michel M, et al. Immune thrombocytopenia in adults: a prospective cohort study of clinical features and predictors of outcome. Haematologica. 2016;101(9):1039–1045. doi: 10.3324/haematol.2016.146373
- Moulis G, Germain J, Comont T, et al. Newly diagnosed immune thrombocytopenia adults: clinical epidemiology, exposure to treatments, and evolution. Results of the CARMEN multicenter prospective cohort. Am J Hematol. 2017;92(6):493–500. doi: 10.1002/ajh.24702
- Liu Y, Chen S, Sun Y, et al. Clinical characteristics of immune thrombocytopenia associated with autoimmune disease: a retrospective study. Medicine (Baltimore). 2016;95(50):e5565. doi: 10.1097/MD.0000000000005565
- Zaja F, Volpetti S, Chiozzotto M, et al. Long-term follow-up analysis after rituximab salvage therapy in adult patients with immune thrombocytopenia. Am J Hematol. 2012;87(9):886–889. doi: 10.1002/ajh.23272
- Porcelijn L, Huiskes E, Schipperus M, et al. Lack of detectable platelet autoantibodies is correlated with nonresponsiveness to rituximab treatment in ITP patients. Blood. 2017;129(25):3389–3391. doi: 10.1182/blood-2016-11-751719
- Zhou H, Xu M, Qin P, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541–1547. doi: 10.1182/blood-2014-06-581868
- Liu XG, Bai XC, Chen FP, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107(6):615–623. doi: 10.1007/s12185-018-2445-z
- Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495–520. doi: 10.1016/j.hoc.2013.03.001
- Baglaenko Y, Chang NH, Johnson SR, et al. The presence of anti-nuclear antibodies alone is associated with changes in B cell activation and T follicular helper cells similar to those in systemic autoimmune rheumatic disease. Arthritis Res Ther. 2018;20(1):264. doi: 10.1186/s13075-018-1752-3
- Chauhan R, Jain D, Dorwal P, et al. The incidence of immunofluorescence patterns and specific autoantibodies observed in autoimmune patients in a tertiary care centre. Eur Ann Allergy Clin Immunol. 2019;51(4):165–173. doi: 10.23822/EurAnnACI.1764-1489.93
- Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73(1):17–23. doi: 10.1136/annrheumdis-2013-203863
- Altintas A, Ozel A, Okur N, et al. Prevalence and clinical significance of elevated antinuclear antibody test in children and adult patients with idiopathic thrombocytopenic purpura. J Thromb Thrombolysis. 2007;24(2):163–168. doi: 10.1007/s11239-007-0031-y
- Abbasi SY, Milhem M, Zaru L. A positive antinuclear antibody test predicts for a poor response to initial steroid therapy in adults with idiopathic thrombocytopenic purpura. Ann Hematol. 2008;87(6):459–462. doi: 10.1007/s00277-008-0448-1
- Li Y, Shi Y, He Z, et al. The efficacy and safety of low-dose rituximab in immune thrombocytopenia: a systematic review and meta-analysis. Platelets. 2019;30(6):690–697. doi: 10.1080/09537104.2019.1624706
- Marangon M, Vianelli N, Palandri F, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol. 2017;98(4):371–377. doi: 10.1111/ejh.12839
- Zaja F, Vianelli N, Volpetti S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–334. doi: 10.1111/j.1600-0609.2010.01486.x
- Sui T, Zhang L, Zhou ZP, et al. Efficacy and safety of two different low-dose rituximab regimens for Chinese adult patients with immune thrombocytopenia. Zhonghua Xue Ye Xue Za Zhi. 2011;32(9):583–586.
- Feng R, Liu XG, Zhao YJ, et al. GPIIb/IIIa autoantibody predicts better rituximab response in ITP. Brit J Haematol. 2018;182(2):305–307. doi: 10.1111/bjh.14782
- Brah S, Chiche L, Fanciullino R, et al. Efficacy of rituximab in immune thrombocytopenic purpura: a retrospective survey. Ann Hematol. 2012;91(2):279–285. doi: 10.1007/s00277-011-1283-3
- Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–239. doi: 10.1111/j.1365-2141.2004.04889.x
- Lucchini E, Zaja F, Bussel J. Rituximab in the treatment of immune thrombocytopenia: what is the role of this agent in 2019? Haematologica. 2019;104(6):1124–1135. doi: 10.3324/haematol.2019.218883
- McKenzie CG, Guo L, Freedman J, et al. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br J Haematol. 2013;163(1):10–23. doi: 10.1111/bjh.12480
- Cooper N, Stasi R, Cunningham-Rundles S, et al. Platelet-associated antibodies, cellular immunity and FCGR3a genotype influence the response to rituximab in immune thrombocytopenia. Br J Haematol. 2012;158(4):539–547. doi: 10.1111/j.1365-2141.2012.09184.x
- Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–451. doi: 10.1038/nri3857
- Gudbrandsdottir S, Brimnes M, Kollgaard T, et al. Effects of rituximab and dexamethasone on regulatory and proinflammatory B-cell subsets in patients with primary immune thrombocytopenia. Eur J Haematol. 2018;100(1):45–52. doi: 10.1111/ejh.12978
- Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–1150. doi: 10.1182/blood-2007-12-129262
- Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 2011;93(1):91–98. doi: 10.1007/s12185-010-0753-z
- Perera M, Garrido T. Advances in the pathophysiology of primary immune thrombocytopenia. Hematology. 2017;22(1):41–53. doi: 10.1080/10245332.2016.1219497