ABSTRACT
Objectives: Although dental caries has been widely reported in individuals with sickle cell disease (SCD), there is still controversial in the literature regarding the association between SCD and dental caries. The aim of this systematic review was to investigate whether individuals with SCD have more dental caries than individuals with non-SCD.
Methods: PubMed and Embase databases were searched for eligible studies. The parameters of the permanent decayed, missing and filled teeth (DMFT) index and the permanent decayed, missing and filled surface (DMFS) index were considered as outcome measures. The overall meta-analyses of the DMFT and DMFS index and various subgroup analyses (caries components, age, and genotypes) of DMFT index were performed to calculate the weighted mean differences (WMD) between patients with SCD and non-SCD individuals.
Results: A total of 9 studies covering 1478 individuals were included in this meta-analysis. The results of overall meta-analyses indicated that the scores of the DMFT and DMFS index were not significantly different between patients with SCD and non-SCD participants. The results of subgroup analyses by caries components, age, and genotypes showed no significant difference in most items. The result of the missing teeth was significantly lower in patients with SCD than in non-SCD individuals (WMD, −0.14; 95% confidence interval [CI], −0.25 to −0.03; P = 0.01).
Discussion and Conclusions: The results revealed that compared with non-SCD individuals, patients with SCD did not suffer from worse dental caries. Considering the limitations, further well-designed studies are necessary to reveal the association between SCD and dental caries.
Introduction
Sickle cell disease (SCD) is one of the most prevalent hemoglobinopathies associated with the replacement of normal hemoglobin with sickle hemoglobin S (HbS) [Citation1–3]. SCD is recognized as a public health issue worldwide that has affected at least 20–25 million people worldwide [Citation4]. As a chronic progressive disease, SCD is a chronic vascular disorder that leads to hemolytic anemia, ongoing organ damage and susceptibility to infection, which are related to high childhood mortality, a considerable financial burden and low quality of life [Citation5–7]. The characteristics of chronic low inflammation of SCD and drug treatments for the disease are considered to be associated with several oral manifestations [Citation8].
Common oral manifestations have been described in SCD patients, including lower salivary flow [Citation9,Citation10], increased levels of biofilm [Citation11,Citation12], hypomineralization of the enamel [Citation13] and dental caries [Citation6,Citation14]. In addition, frequent admission, drug treatments, and diminished oral hygiene are high-risk factors for caries, which contribute to more dental caries in SCD patients [Citation15–17]. Dental caries can destroy hard tissues of the teeth and may lead to pulpitis and periapical periodontitis, which can even become a source of infection in sickle cell crisis due to an insufficient blood supply [Citation18,Citation19]. Dental caries is a common oral manifestation in individuals with SCD [Citation20,Citation21]. Moreover, the association between SCD and dental caries has aroused great interest from clinical doctors and researchers.
Recently, Al-Alawi found significantly higher numbers of decayed teeth among SCD individuals compared with non-SCD individuals [Citation15]. Brandao observed reduced salivary flow in individuals with SCD who had more dental caries than non-SCD controls [Citation9]. However, several other investigators have not noted any statistically significant results in the comparison of dental caries between groups. Therefore, there is a lack of consensus in the literature regarding the association between SCD and dental caries [Citation22,Citation23]. This inconclusive evidence is not advantageous for facilitating oral hygiene management and the prevention of dental caries among SCD individuals.
Therefore, it is necessary to summarize current evidence on the association of dental caries in those with SCD for preventive policies against dental caries. We conducted this systematic review and meta-analysis to evaluate the association between SCD and dental caries.
Materials and methods
Guidelines and registration
We conducted and reported this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation24] and registered it in PROSPERO (CRD42019132139).
Focused question
Is there any relationship between SCD and dental caries?
Literature search
We conducted an electronic search of PubMed and Embase up to August 2019. We used the following search terms: (1) ‘sickle cell anemia’ or ‘sickle cell diseases’, and (2) ‘dental caries’ or ‘oral manifestation’ or ‘oral health’. In addition, we also identified additional citations by reviewing the reference lists of included studies and pertinent reviews. We contacted the original authors through e-mail for extra data if necessary. The detailed search strategy is illustrated in supplementary file S1.
Inclusion and exclusion criteria
Studies were included if they (1) met a cohort, case-control, or cross-sectional study design and were published in English; (2) diagnosed with or without SCD irrespective of ages; (3) had clear diagnostic criteria for SCD; (4) reported measures of dental caries such as the decayed, missing and filled permanent/deciduous teeth (DMFT/dmft) index; the decayed, missing and filled permanent/deciduous surface (DMFS/dmfs) index; and the decayed permanent/deciduous teeth (D/d), the missing permanent/deciduous teeth (M/m) and the filled permanent/deciduous teeth (F/f) indexes between patients with SCD and non-SCD participants; and (5) data on measures of dental caries were analyzed and were expressed as continuous variables.
Reviews, case reports, and studies provided unavailable, overlapping data and original data expressed as figures were excluded.
Study selection
To select the studies, two reviewers first excluded duplicate studies. After scrutinizing the titles and abstracts of all identified studies, we excluded apparently ineligible studies and selected the potential eligible ones. Then, two reviewers read full papers carefully and analyzed the inclusion and exclusion criteria for further eligible studies. After completing the selection, we included all eligible studies in which the investigators reported measures of dental caries between patients with SCD and non-SCD participants. During the selection and eligibility screening process, two reviewers independently assessed the studies and we resolved any disagreements by means of discussion. If we could not reach a consensus, a third reviewer decided whether the study would be included.
Data extraction
Two reviewers independently performed data extraction from each of the eligible studies. A third reviewer checked the result for accuracy. The main information is as follows: family name of the first author; year of publication; study country; study design; age and number of enrolled participants; diagnostic methods of SCD; outcomes of measuring dental caries; and P values.
Quality assessment
Two reviewers used the Newcastle-Ottawa scale (NOS) to evaluate the methodological quality of cohort studies and case-control studies [Citation25,Citation26]. This assessment scale comprises selection, comparability, and outcome. We used the three broad categories to appraise the quality of the cohort and case-control studies, with a maximum of 9 points. For cross-sectional studies, we used the Agency for Healthcare Research and Quality (AHRQ) recommended criteria to evaluate the methodological quality [Citation27]. The criteria use 11 items, and each item has an answer of either ‘YES’, ‘NO’ or ‘UNCLEAR’. We would score ‘0’ if the item was answered 'NO' or ‘UNCLEAR’ and we would score ‘1’ if the item was answered ‘YES’. Two (items d and k) of the 11 items in the AHRQ recommended criteria were not suitable in our study. The cross-sectional studies also scored a maximum of 9 points. Two reviewers independently completed the quality assessment of the included studies, and we resolved any disagreements by means of discussion. If we could not reach a consensus, a third (Bo Hu) reviewer would decide the outcome.
Statistical analysis
We used software (RevMan 5.3, Cochrane Collaboration) to perform statistical analysis of the included studies. We pooled the weighted mean difference (WMD) with a confidence interval (CI) of 95% from each study to explain the relationships between SCD and dental caries measured by the DMFT or DMFS indexes. We used I2 to estimate between-study heterogeneity [Citation28]. An I2 of more than 75.0% indicated high heterogeneity; an I2 of between 50.0% and 75.0% indicated moderate heterogeneity, and an I2 of less than 50.0% indicated low heterogeneity. We used the fixed-effect model when low heterogeneity was found. Otherwise, we used a random-effect model. Sensitivity analysis was conducted by the exclusion of any single study in turn to analyze the stability of the pooled results, if at least 5 studies were included in the comparison. Funnel plots were used to assess any potential publication bias in at least 10 studies.
Analysis of subgroups
We conducted a subgroup analysis by components of the DMFT indexes to calculate the WMDs between patients with SCD and non-SCD participants. We conducted subgroup analyses by each item of the DMFT indexes to further identify the association between SCD and dental caries. We used subgroup analyses of DMFT by age and genotype of the SCD groups and explored the potential source of heterogeneity. First, we divided the patients with SCD into 2 age groups: children/adolescents and adults. Second, in some of the included studies, the investigators divided patients with SCD into different genotype groups. So, we divided the patients with SCD into 2 common genotype groups: the HbSS genotype and HbSC genotype.
Results
Study selection
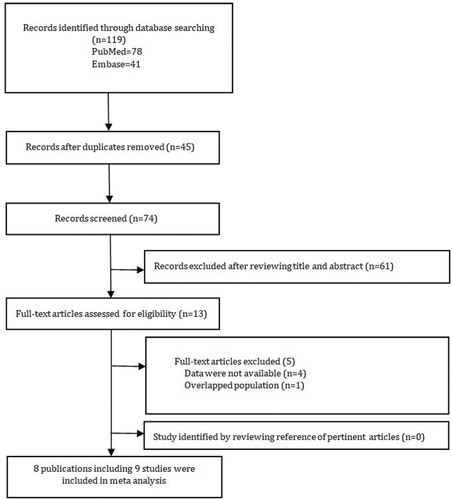
illustrates the flow diagram of the study selection process. According to our search strategy, we comprehensively retrieved and identified 78 and 41 articles from PubMed and Embase, respectively. We reviewed 13 full-text articles; however, the investigators of one of the 4 studies did not provide available data [Citation11,Citation16,Citation29,Citation30]. Although we sent emails to contact the corresponding authors of these studies, we received no reply. Moreover, we noted that 2 publications were the same by Fernandes but provided case arms for children and teens separately, and data from the matched control group were used in more than one comparison [Citation22,Citation31]. Ultimately, we included a total of 9 studies within 8 publications featuring a comparison group included in the systematic review and in the meta-analysis [Citation9,Citation15,Citation16,Citation22,Citation23,Citation32–34].
Summary of the included studies
summarizes the main characteristics of the included studies [Citation9,Citation15,Citation16,Citation22,Citation23,Citation32–34]. The publication year ranged from 2002 to 2018. The included publications were 6 cross-sectional [Citation9,Citation15,Citation16,Citation22,Citation23,Citation33] and 2 retrospective cohort studies [Citation32,Citation34]. The investigators conducted three studies [Citation32–34] in the USA, four [Citation9,Citation16,Citation22,Citation23] in Brazil, and one [Citation15] in Saudi Arabia. The investigators evaluated a total of 545 patients with SCD and 967 non-SCD healthy control participants. No study reported the dmfs index. The authors of one study reported the dmft index and found that patients with SCD had more significantly scores than non-SCD children controls [Citation9]. Authors of six [Citation9,Citation15,Citation22,Citation23,Citation33,Citation34] studies reported the scores of the DMFT index; the same authors of two cohort studies [Citation32,Citation34] reported the scores of the DMFS index and authors of one study [Citation34] reported the scores of both the DMFT index and DMFS index; authors of one study only reported the scores of decayed teeth. Investigators in most of the included studies [Citation9,Citation15,Citation16,Citation22,Citation23] used the World Health Organization (WHO) criteria to measure the DMFT index. Investigators in two studies used retrospective records to measure the DMFS index [Citation32,Citation34].
Table 1. Characteristics of the studies included.
Quality assessment
We evaluated the quality of the 2 cohort studies [Citation32,Citation34] using NOS and found a score of 7 in the cohort studies; we thus considered them to be high-quality studies. We assessed the quality of the 6 cross-sectional studies [Citation9,Citation15,Citation16,Citation22,Citation23,Citation33] by the AHRQ recommended criteria. For item ‘a’ and item ‘g’, all answers were ‘YES’. For items ‘b’ and ‘h’, all answers were ‘YES’. For item e, all answers were ‘NO’. We found a score of 5 in 3 studies and a score of 6 in 3 studies. We considered them to be moderate- and high-quality studies. The methodological assessment evaluation of the cohort studies and the cross-sectional studies shows in and , respectively.
Table 2. Methodological quality scores of the selected articles.
Table 3. Methodological quality scores of the selected articles.
Meta-analysis
Investigators of 5 studies [Citation9,Citation15,Citation22,Citation33,Citation34] including a total of 673 non-SCD healthy control participants and 289 patients with SCD estimated the relationship between dental caries and SCD measured by levels in the DMFT index. Investigators of 2 cohort studies [Citation32,Citation34] including a total of 243 non-SCD healthy control participants and 137 patients with SCD estimated the relationship between dental caries and SCD measured by levels in the DMFS index. The results of separate meta-analyses indicated that the DMFT and DMFS scores were not significantly different between patients with SCD and non-SCD healthy control participants (WMD, −0.11; 95% CI, −0.47, 0.26; P = 0.56; and WMD, −0.77; 95% CI, −4.10, 5.64; P = 0.20, respectively). Considering the low heterogeneity of the DMFS index (I2 = 29%) between studies, we selected a fixed-effects model for the meta-analysis. However, we considered the heterogeneity between the studies of the DMFT index to be moderate (I2 = 61%), so we conducted a random-effects model for the meta-analysis. When conducting a sensitivity analysis by the exclusion of any single study in turn from the meta-analysis, the results remained significantly unchanged, indicating that the meta-analysis of the DMFT is robust. The information on the results of the meta-analysis evaluating DMFT and DMFS is provided in and , respectively.
Figure 2. Forest plot presenting weighted mean differences (WMD) of the permanent decayed, missing and filled teeth (DMFT) between patients with SCD and non-SCD participants. CI: Confidence interval. SCD: Sickle cell disease. Non-SCD: participants without sickle cell disease.
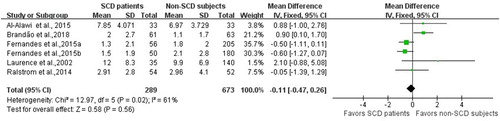
Figure 3. Forest plot presenting weighted mean differences (WMD) of the permanent decayed, missing and filled surface (DMFS) between patients with SCD and non-SCD participants. CI: Confidence interval. SCD: Sickle cell disease. Non-SCD: participants without sickle cell disease.
The information on the results of the subgroup analyses is provided in . We conducted a subgroup analysis by each item of the DMFT index. Investigators of 3 studies [Citation9,Citation15,Citation22] reported the permanent missing teeth (M) index. The results of the meta-analysis showed that patients with SCD had lower M scores than non-SCD participants (WMD, −0.14; 95% CI, −0.25, −0.03). Investigators of four articles [Citation9,Citation15,Citation16,Citation22] reported the D index and three articles [Citation9,Citation15,Citation22] evaluating the F index were included in the study. However, no statistically significant difference between individuals with SCD and non-SCD was found for scores of the D index (WMD, 1.14; 95% CI, −0.04 to 2.33) and the F index (WMD, −0.22; 95% CI, −0.66 to 0.21) of the DMFT index. In addition, the sensitivity analysis of the D index was stable. We selected a random-effects model for subgroup analyses by each item of the DMFT index.
Table 4. Results of subgroup analyses.
We conducted a subgroup analysis of the DMFT index by age group of the participants (children/adolescents, adults). There was no significant difference for the subgroup of DMFT in SCD patients (children/adolescents: WMD = −0.26, 95% CI, −0.64, 0.12, adults: WMD, 1.23; 95% CI, −0.36, 2.82).
We conducted a subgroup analysis of DMFT by two genotypes of SCD individuals. Four studies [Citation22,Citation23,Citation33,Citation34] reported the HbSS genotype of SCD patients, and two studies [Citation23,Citation33] reported the HbSC genotype of SCD patients in evaluating the DMFT index compared to that of non-SCD subjects. No significant difference was observed for the subgroups of DMFT in SCD patients with genotype HbSS (WMD, −0.42; 95% CI, −0.84, −0.00) or HbSC (WMD, −1.17; 95% CI, −2.47, −0.13) compared with non-SCD subjects, which showed low heterogeneity between the studies of the DMFT index in the HbSS and HbSC genotypes in SCD patients. A fixed-effects model was conducted. The sensitivity analysis revealed that the results are stable.
Discussion
This systematic review assessed the association between dental caries and SCD in affected individuals. Dental caries is a chronic and destructive condition of dental tissues and may lead to the complications of pulpitis, periapical periodontitis and acute pain, which, if left untreated, may even lead to loss of teeth [Citation35]. Dental caries remains a disease associated with significant health effects, financial costs and low quality of life. Dental caries is one of the dental manifestations commonly reported in individuals with SCD. The complications of pulpitis and periapical periodontitis due to insufficient blood supply may become a source of infection in sickle cell crisis in SCD individuals [Citation18,Citation19]. Recently, the association between dental caries and SCD has sparked great interest among dental researchers and affected patients. However, there are inconsistencies based on the current research findings [Citation9,Citation15,Citation16,Citation22,Citation23,Citation32–34]. This inconclusive evidence may hinder oral hygiene management and the prevention of dental caries in individuals with SCD. Therefore, we conducted a systematic scientific review and meta-analysis in an attempt to elucidate the association between SCD and dental caries.
The DMFT/DMFS index represents caries severity and prevalence, which is considered a lifetime cumulative burden of dental caries [Citation36]. Individuals with SCD commonly reported hypomineralization of enamel, diminished oral hygiene, and low salivary flow rate, and drug treatments containing sugar were found in SCD patients [Citation17,Citation37], suggesting that the association of risk factors may lead to susceptibility to dental caries in these affected individuals. However, in this systematic review, no statistically significant differences were found in the scores of dental caries between SCD patients and the non-SCD group as measured by the mean DMFT and DMFS indexes. On the one hand, SCD patients are usually asked to drink plenty of water to prevent sickle cell pain crisis, possibly leading to fluoride exposure through the water supply due to public health policies in some regions [Citation33]. Fluoridated water may promote the remineralization of dental enamel and neutralization of acid substances, which is an effective measure for the prevention of caries disease [Citation38]. On the other hand, SCD patients may have lower sugar consumption in the diet for fear of weakening their blood [Citation21]. Additionally, lower sugar intake can reduce the development of dental caries [Citation39].
The components of the DMFT index consist of the decayed, missing and filled teeth. And we conducted subgroup analyses of components of the DMFT index. No significant difference was found in most of the components, apart from the M index. Although the result of the M index was significantly lower in patients with SCD than in non-SCD participants, we believe that this result may exhibit bias. Authors of six [Citation9,Citation15,Citation22,Citation23,Citation33,Citation34] reported the scores of the DMFT index, three of which reported the M index. One study reported a higher M index in the SCD group but no difference was found [Citation15]. Two of the studies reported lower M indices in SCD patients, one of which was not significantly different [Citation9], and the other one was significantly different [Citation22]. We carefully analyzed the article with statistical significance and found that the investigators of the study concluded that the reason for lower M is that SCD patients had free access to oral health care provided by the government in the regions [Citation22]. This may be the reason for the reduction of dental caries. Therefore, interpretation of the meaningfulness of the M in this meta-analysis should be considered with caution.
Both SCD and dental caries are characterized by chronic and progressive conditions, and they may share common confounders, such as age and economic status [Citation40]. Although understanding the association between SCD and non-SCD subjects from different economic statuses could be beneficial, due to the insufficient information provided by the included studies, we could not assess the effects of the economic status on the overall effects. Considering that 6 studies focused on different age groups of participants, ranging from 3 to 68 years old in our meta-analysis, dental caries and SCD were found to be associated with age groups [Citation22]. Therefore, we conducted an age subgroup analysis to further evaluate dental caries in SCD patients and non-SCD subjects. In the analysis of DMFT by subgroups of children/adolescents and adults, non-SCD children/adolescents did not have significantly higher scores than children/adolescents with SCD. No statistically significant difference between SCD and non-SCD adults was found for DMFT.
SCD is a disease that is a group of autosomal recessive disorders, including hemoglobin SS disease (HbSS genotype), sickle cell hemoglobin C disease (HbSC genotype), and homozygous beta-thalassemia [Citation3]. HbSS and HbSC are the most common genotypes of SCD, and the former is the most severe form. Studies have shown that the clinical severity or genotype of SCD (HbSS or HbSC) may be associated with dental caries [Citation34,Citation41]. Therefore, we conducted a subgroup analysis of SCD genotypes using the DMFT index. In the analysis of DMFT by subgroups of HbSS and HbSC, the subgroups of individuals with SCD with the genotype HbSS and genotype HbSC showed that these affected people exhibited a significant difference in the DMFT index compared to those without SCD.
In this paper, moderate levels of heterogeneity between studies were found (Pheterogeneity = 0.02, I2 = 61%) in the overall meta-analysis of the DMFT index; therefore, we used a random-effects model in the comparison. The variation in results among studies may be due to there were multiple confounders, including different countries with different socioeconomic factors, especially, the inconformity of age, as well as the genotypes of SCD (HbSS and HbSC). First, dental caries is associated with different age groups. Thus, we performed a subgroup analysis of DMFT by age group (children/adolescents and adults). Additionally, according to the change in heterogeneity compared with the overall analysis, we could not explain the source of heterogeneity from the age groups. Second, we conducted a subgroup analysis for genotypes of SCD. We found a decrease in the level of heterogeneity from moderate to low (HbSS, I2 = 0%, P = 0.41; HbSC, I2 = 17%, P = 0.27) upon the subgroup analysis, indicating that genotypes of SCD may explain mainly the observed heterogeneity across studies. Additionally, a mild heterogeneity still existed (HbSC, I2 = 17%). This finding may be explained by different economic statuses. Despite all of the above confounding factors, the heterogeneity of our meta-analyses is acceptable.
To the best of our knowledge, this systematic review and meta-analysis elucidate for the first time the current evidence on associations between SCD and dental caries. In this meta-analysis, the effect of the DMFT and DMFS indexes were analyzed to compare dental caries between the SCD and non-SCD groups. In addition, for the analysis of DMFT, subgroups of age and genotype of SCD participants were further evaluated, which provides more information for understanding the relationship between SCD and dental caries. Simultaneously, several limitations of the present study should be acknowledged. First, moderate levels of heterogeneity were found; therefore, we used a random-effect model in the comparison of some indexes. In addition, the sensitivity analysis revealed that the results of the DMFT index are stable. And subgroup analyses of DMFT by age and genotype of SCD were conducted. Second, because of the limited number of papers, we could not evaluate publication bias. Third, due to the limited information provided by the included studies, we could not analyze the effects of the common risk factors (such as health/dental care systems, socioeconomic and behavioral factors) shared by dental caries and SCD on the overall effects. More well-designed longitudinal studies are needed in the future to clarify the association between dental caries in SCD patients.
In conclusion, the results of the studies revealed that patients with SCD did not suffer from worse dental caries than did non-SCD healthy control participants. In addition, the subgroup analyses of DMFT by age and genotype of the SCD groups of participants failed to show a significant difference in the association between SCD and dental caries. Considering the limitations of the current study, the interpretation of the meaningfulness of this meta-analysis should be considered with caution. Therefore, it is still necessary to pay constant attention to SCD patients’ dental health in clinical work. More well-designed longitudinal studies evaluating the association between SCD and dental caries are encouraged.
Supplemental Material
Download MS Word (14.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood & Cancer. 2012;59(2):386–390. doi:10.1002/pbc.24175. PubMed PMID: 22535620. doi: 10.1002/pbc.24175
- Bunn HF. Pathogenesis and treatment of sickle cell disease. The New England Journal of Medicine. 1997;337(11):762–769. Epub 1997/09/11. doi:10.1056/nejm199709113371107. PubMed PMID: 9287233. doi: 10.1056/NEJM199709113371107
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet (London, England). 2010;376(9757):2018–2031. Epub 2010/12/07. doi:10.1016/s0140-6736(10)61029-x. PubMed PMID: 21131035. doi: 10.1016/S0140-6736(10)61029-X
- Modell B. Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization. 2008;2008(6):480–487. doi:10.2471/blt.06.036673.
- Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet (London, England). 2013;381(9861):142–151. Epub 2012/10/30. doi:10.1016/s0140-6736(12)61229-x. PubMed PMID: 23103089; PubMed Central PMCID: PMCPMC3547249. doi: 10.1016/S0140-6736(12)61229-X
- Luna AC, Rodrigues MJ, Menezes VA, et al. Caries prevalence and socioeconomic factors in children with sickle cell anemia. Brazilian Oral Research. 2012;26(1):43–49. Epub 2012/02/22. PubMed PMID: 22344337. doi: 10.1590/S1806-83242012000100008
- Athale UH, Chintu C. Clinical analysis of mortality in hospitalized Zambian children with sickle cell anaemia. East African Medical Journal. 1994;71(6):388–391. Epub 1994/06/01. PubMed PMID: 7835262.
- Kawar N, Alrayyes S, Aljewari H. Sickle cell disease: an overview of orofacial and dental manifestations. Disease-a-Month: DM. 2018;64(6):290–295. Epub 2018/01/18. doi:10.1016/j.disamonth.2017.12.004. PubMed PMID: 29338872. doi: 10.1016/j.disamonth.2017.12.004
- Brandao CF, Oliveira VMB, Santos A, et al. Association between sickle cell disease and the oral health condition of children and adolescents. BMC Oral Health. 2018;18(1):169. Epub 2018/10/22. doi: 10.1186/s12903-018-0629-9. PubMed PMID: 30342522; PubMed Central PMCID: PMCPMC6196017. doi: 10.1186/s12903-018-0629-9
- de Matos BM, Ribeiro ZE, Balducci I, et al. Oral microbial colonization in children with sickle cell anaemia under long-term prophylaxis with penicillin. Archives of Oral Biology. 2014;59(10):1042–1047. Epub 2014/06/27. doi:10.1016/j.archoralbio.2014.05.014. PubMed PMID: 24967510. doi: 10.1016/j.archoralbio.2014.05.014
- Singh J, Singh N, Kumar A, et al. Dental and periodontal health status of Beta thalassemia major and sickle cell anemic patients: a comparative study. J Int Oral Health: JIOH. 2013;5(5):53–58. Epub 2013/12/11. PubMed PMID: 24324305; PubMed Central PMCID: PMCPMC3845285.
- Guzeldemir E, Toygar HU, Boga C, et al. Dental and periodontal health status of subjects with sickle cell disease. Journal of Dental Sciences. 2011;6(4):227–234. https://doi.org/10.1016/j.jds.2011.09.008.
- Taylor LB, Nowak AJ, Giller RH, et al. Sickle cell anemia: a review of the dental concerns and a retrospective study of dental and bony changes. Special Care in Dentistry: Official Publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry. 1995;15(1):38–42. Epub 1995/01/01. PubMed PMID: 7676364. doi: 10.1111/j.1754-4505.1995.tb00469.x
- Laurence B, Woods D, George D, et al. Self-perceived loss of control and untreated dental decay in African American adults with and without sickle cell disease. Journal of Health Care for the Poor and Underserved. 2006;17(3):641–651. Epub 2006/09/09. doi:10.1353/hpu.2006.0107. PubMed PMID: 16960327. doi: 10.1353/hpu.2006.0107
- Al-Alawi H, Al-Jawad A, Al-Shayeb M, et al. The association between dental and periodontal diseases and sickle cell disease. A pilot case-control study. The Saudi Dental Journal. 2015;27(1):40–43. Epub 2014/12/30. doi:10.1016/j.sdentj.2014.08.003. PubMed PMID: 25544813; PubMed Central PMCID: PMCPMC4273253. doi: 10.1016/j.sdentj.2014.08.003
- Kalbassi S, Younesi MR, Asgary V. Comparative evaluation of oral and dento-maxillofacial manifestation of patients with sickle cell diseases and beta thalassemia major. Hematology (Amsterdam, Netherlands). 2018;23(6):373–378. Epub 2017/11/23. doi:10.1080/10245332.2017.1404219. PubMed PMID: 29165026.
- Crawford JM. Periodontal disease in sickle cell disease subjects. Journal of Periodontology. 1988;59(3):164–169. Epub 1988/03/01. doi:10.1902/jop.1988.59.3.164. PubMed PMID: 3162981. doi: 10.1902/jop.1988.59.3.164
- O'Rourke C, Mitropoulos C. Orofacial pain in patients with sickle cell disease. British Dental Journal. 1990;169(5):130–132. Epub 1990/09/08. PubMed PMID: 2206667. doi: 10.1038/sj.bdj.4807296
- Rada RE, Bronny AT, Hasiakos PS. Sickle cell crisis precipitated by periodontal infection: report of two cases. Journal of the American Dental Association (1939). 1987;114(6):799–801. doi:10.14219/jada.archive.1987.0173.
- Smith HB, McDonald DK, Miller RI. Dental management of patients with sickle cell disorders. Journal of the American Dental Assocication (1939). 1987;114(1):85–87. Epub 1987/01/01. PubMed PMID: 2948994. doi: 10.14219/jada.archive.1987.0055
- Okafor LA, Nonnoo DC, Ojehanon PI, et al. Oral and dental complications of sickle cell disease in Nigerians. Angiology. 1986;37(9):672–675. doi: 10.1177/000331978603700909
- Fernandes ML, Kawachi I, Corrêa-Faria P, et al. Caries prevalence and impact on oral health-related quality of life in children with sickle cell disease: cross-sectional study. BMC Oral Health. 2015;15:68. doi:10.1186/s12903-015-0052-4.
- Passos CP, Santos PRB, Aguiar MC, et al. Sickle cell disease does not predispose to caries or periodontal disease. Special Care in Dentistry. 2012;32(2):55–60. doi:10.1111/j.1754-4505.2012.00235.x.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology. 2009;62(10):1006–1012. Epub 2009/07/28. doi:10.1016/j.jclinepi.2009.06.005. PubMed PMID: 19631508. doi: 10.1016/j.jclinepi.2009.06.005
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. Epub 2010/07/24. doi:10.1007/s10654-010-9491-z. PubMed PMID: 20652370. doi: 10.1007/s10654-010-9491-z
- Wells GA SB, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta analyses; 2009.
- Rostom A DC, Cranney A, Saloojee N, et al. Appendix D. Quality assessment forms. In: Celiac disease. Agency for Healthcare Research and Quality (US); 2004. https://wwwncbinlmnihgov/books/NBK35156/.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed). 2003;327(7414):557–560. Epub 2003/09/06. doi:10.1136/bmj.327.7414.557. PubMed PMID: 12958120; PubMed Central PMCID: PMCPMC192859. doi: 10.1136/bmj.327.7414.557
- Okafor LA, Nonnoo DC, Ojehanon PI, et al. Oral and dental complications of sickle cell disease in Nigerians. Angiology. 1986;37(9):672–675. Epub 1986/09/01. doi:10.1177/000331978603700909. PubMed PMID: 3767074. doi: 10.1177/000331978603700909
- Laurence B, Woods D, George D, et al. Self-perceived loss of control and untreated dental decay in African American adults with and without sickle cell disease. Journal of Health Care for the Poor Underserved. 2006;17(3):641–651. doi:10.1353/hpu.2006.0107.
- da Matta Felisberto Fernandes ML, Kawachi I, Fernandes AM, et al. Oral health-related quality of life of children and teens with sickle cell disease. Revista brasileira de hematologia e hemoterapia. 2016;38(2):106–112. Epub 2016/05/22. doi:10.1016/j.bjhh.2016.01.004. PubMed PMID: 27208568; PubMed Central PMCID: PMCPMC4877612. doi: 10.1016/j.bjhh.2016.01.004
- Laurence B, George D, Woods D, et al. The association between sickle cell disease and dental caries in African Americans. Special Care in Dentistry: Official Publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry. 2006;26(3):95–100. Epub 2006/06/16. PubMed PMID: 16774185; PubMed Central PMCID: PMCPMC1786275. doi: 10.1111/j.1754-4505.2006.tb01430.x
- Ralstrom E, da Fonseca MA, Rhodes M, et al. The impact of sickle cell disease on oral health-related quality of life. Pediatric Dentistry. 2014;36(1):24–28. Epub 2014/04/11. PubMed PMID: 24717705.
- Laurence B, Reid BC, Katz RV. Sickle cell anemia and dental caries: a literature review and pilot study. Special Care in Dentistry: Official Publication of the American Association of Hospital Dentists, the Academy of Dentistry for the Handicapped, and the American Society for Geriatric Dentistry. 2002;22(2):70–74. Epub 2002/07/12. PubMed PMID: 12109598. doi: 10.1111/j.1754-4505.2002.tb01165.x
- Li R, Zhao Y, Ye L. How to make choice of the carious removal methods, Carisolv or traditional drilling? A meta-analysis. Journal of Oral Rehabilitation. 2014;41(6):432–442. Epub 2014/03/26. doi:10.1111/joor.12161. PubMed PMID: 24661083. doi: 10.1111/joor.12161
- Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent. 2005;33(3):223–233. Epub 2005/02/24. doi:10.1016/j.jdent.2004.10.009. PubMed PMID: 15725522. doi: 10.1016/j.jdent.2004.10.009
- Lopes CMI, Cavalcanti MC, Alves ELAC, et al. Enamel defects and tooth eruption disturbances in children with sickle cell anemia. Brazilian Oral Research. 2018;32:e87), Epub 2018/08/16. doi:10.1590/1807-3107bor-2018.vol32.0087. PubMed PMID: 30110085. doi: 10.1590/1807-3107bor-2018.vol32.0087
- Garcia-Godoy F, Hicks MJ. Maintaining the integrity of the enamel surface: the role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. Journal of the American Dental Association (1939). 2008;139(Suppl):25s–34s. Epub 2008/07/03. PubMed PMID: 18460677. doi: 10.14219/jada.archive.2008.0352
- Marshall TA, Eichenberger-Gilmore JM, Larson MA, et al. Comparison of the intakes of sugars by young children with and without dental caries experience. Journal of the American Dental Association (1939). 2007;138(1):39–46. Epub 2007/01/02. PubMed PMID: 17197400. doi: 10.14219/jada.archive.2007.0019
- Schwendicke F, Dorfer CE, Schlattmann P, et al. Socioeconomic inequality and caries: a systematic review and meta-analysis. Journal of Dental Research. 2015;94(1):10–18. Epub 2014/11/15. doi:10.1177/0022034514557546. PubMed PMID: 25394849. doi: 10.1177/0022034514557546
- Fernandes ML, Kawachi I, Correa-Faria P, et al. The impact of the oral condition of children with sickle cell disease on family quality of life. Brazilian Oral Research. 2016;30, Epub 2016/02/26. doi:10.1590/1807-3107BOR-2016.vol30.0021. PubMed PMID: 26910017.