ABSTRACT
Background: Serum IgG4 is typically measured to investigate for Immunoglobulin G4-related Disease (IgG4-RD), a fibroinflammatory condition associated with polyclonal increase in serum IgG4. However, increased IgG4 can also be monoclonal, and little is known about IgG4 myeloma. Methods: We describe two cases of IgG4 myeloma without clinical, radiologic, or laboratory features of IgG4-related disease. Results: An 84 year old man presented with anemia and compression fractures and a 77 year old man presented with anemia, hypercalcemia and renal failure. Both had markedly elevated monoclonal serum IgG4, 34 g/L and 48 g/L in the beta region, and increased IgG positive bone marrow plasma cells, 50% and 80%, respectively. Neither had clinical or radiological manifestations of IgG4-related disease (IgG4-RD) such as salivary or lacrimal gland swelling, autoimmune pancreatitis , or retroperitoneal fibrosis. Both cases responded well to standard myeloma therapy. The IgG4 paraprotein caused spuriously elevated beta-2 microglobulin of 45.2 mg/L in case two due to interference with the assay. Conclusion: These cases illustrate the importance of performing serum protein electrophoresis in tandem with IgG subclasses to distinguish between polyclonal and monoclonal increases in serum IgG4. The lack of typical IgG4-RD features in these two patients suggests that monoclonal elevation in serum IgG4 alone is insufficient to cause the organ damage characteristic of IgG4-RD. Larger studies of IgG myeloma subtypes are warranted to explore whether IgG1, IgG2, IgG3 and IgG4 myeloma differ in natural history and whether the interference with beta-2 microglobulin is specific to IgG4 monoclonal proteins.
Introduction
IgG4-related disease (IgG4-RD) is a systemic fibroinflammatory condition which was discovered in Japan in the early 2000s and can involve nearly any organ [Citation1,Citation2]. Biopsies from most involved tissues exhibit histologic features of storiform (swirling) fibrosis, obliterative phlebitis, polyclonal lymphoplasmacytic infiltrate enriched with IgG4+ plasma cells and an IgG4+/IgG+ plasma cell ratio of >40% [Citation3]. Serum IgG4 is elevated in approximately 70% of patients with IgG4-RD, although Asian patients tend to have higher serum IgG4 levels than White patients [Citation4,Citation5]. Interest in measuring serum IgG subclasses has increased dramatically over the past 15 years since the advent of IgG4-RD, and IgG subclass measurement is recognized as an important investigation for polyclonal hypergammaglobulinemia [Citation6,Citation7], as well as investigation for eosinophilia [Citation8]. However, despite the presence of IgG4 in serum and tissues, this molecule is not considered pathogenic in the disease. Diagnosis of IgG4-RD therefore should not rely on elevated serum IgG4 levels alone but on clinical and histological findings consistent with the International Consensus Criteria [Citation9], or the recent 2019 ACR/EULAR classification criteria [Citation10].
Ironically, although human IgG subclasses were first discovered by studying IgG monoclonal proteins in myeloma subjects in the 1960s [Citation11–13], there have been very few reports of IgG4-myeloma in the modern era. The few extant cases have not described whether patients have features of IgG4-related disease, nor have they provided a detailed description of other clinical findings, laboratory and radiology features, or response to therapy [Citation14–16]. In this report, we present two cases of plasma cell myeloma with markedly elevated serum concentrations of IgG4, but without overt clinical manifestations of IgG4-RD and partial response to standard myeloma therapy. These cases provide proof of principle that IgG4 is likely a reactive product of immune dysregulation in IgG4-RD rather than the causative molecule and provide an important foundation for future studies of IgG4 myeloma. We also report an interesting phenomenon of IgG4 monoclonal protein interference causing spuriously elevated β2-microglobulin in case 2.
Case presentation: Case 1
An 84-year-old white man presented with a two-month history of muscle weakness and hip pain. His past medical history included left total hip replacement and kyphoplasty for osteoporotic vertebral fractures. Physical examination was unremarkable and no swelling was noted in periorbital and salivary gland areas. Bloodwork revealed hemoglobin 116 g/L, MCV 95 fL, mild rouleaux on blood film, with no eosinophilia (eosinophils 0.1 giga/L); on the serum protein electrophoresis (SPEP) there was a 34 g/L IgG kappa monoclonal protein running in the beta-2 region and marked suppression of polyclonal gamma globulins (). Urine protein electrophoresis showed a small monoclonal IgG kappa band in the beta-2 region and a faint monoclonal-free kappa light chain band in beta-1 region. Beta-2 microglobulin was 2.8 mg/L (reference <2.2 mg/L), C-reactive protein (CRP) 12 mg/L (reference <3.1 mg/L), serum kappa-free light chain concentration 563 mg/L (reference: 3.3-19.4 mg/L), lambda-free light chain 2.9 mg/L (reference 5.7-26.3 mg/L), with a kappa/lambda ratio of 194.14 (reference: 0.25-1.65). Skeletal survey revealed severe multiple compression deformities in the lumbar spine but no lytic lesions. CT scan of the chest, abdomen and pelvis confirmed diffuse osteopenia and multiple lumbar compression fractures but no discrete lytic lesions and no manifestations of IgG4-RD such as chronic pancreatitis, retroperitoneal fibrosis, or lymphadenopathy. IgG subclassification using liquid chromatography with tandem mass spectrometry revealed predominance of the IgG4 subclass at 16.3 g/L (reference: 0.052-1.250 g/L) [Citation17]. Bone marrow aspirate and biopsy revealed kappa-clonal plasma cell myeloma with mature morphology comprising 50% of the cellular marrow. IgG4 staining of the bone marrow specimen, however, was technically difficult precluding its assessment. The plasma cells expressed CD38 and CD138, with aberrant expression of CD56 and CD117 and loss of CD19 and CD81. Additionally, a small lambda-restricted B-cell population was identified by flow cytometry but not histology, consistent with coexisting monoclonal B-cell lymphocytosis. No evidence of 17p deletion or IGH translocation was noted by FISH. Additional laboratory tests revealed no nuclear antibodies and the absence of hepatitis and HIV infection. The patient was diagnosed with IgG4 plasma cell myeloma (R-ISS Stage I) and was started on lenalidomide and dexamethasone. After six cycles of treatment, the patient was clinically well and had a partial response. Subsequent laboratory results are summarized in .
Figure 1. Serum protein electrophoresis in both patients. Case 1 shows a 34 g/L IgG kappa monoclonal protein running in the beta-2 region and marked suppression of polyclonal gamma globulins. Case 2 shows a 48 g/L IgG lambda monoclonal band running in the beta-1 region, a monoclonal-free lambda light chain band in the beta-2 region, and polyclonal decrease in other immunoglobulins.
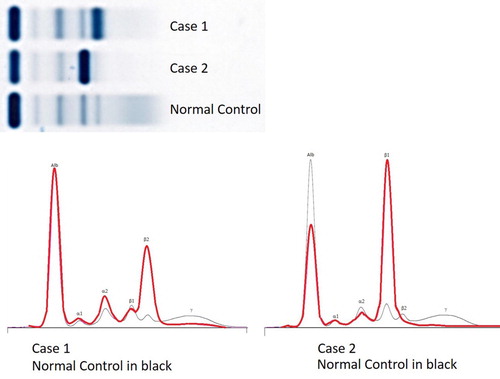
Table 1. Summary of immunoglobulin, IgG4 subclass, monoclonal protein and serum-free light chain concentrations for the two cases.
Case presentation: Case 2
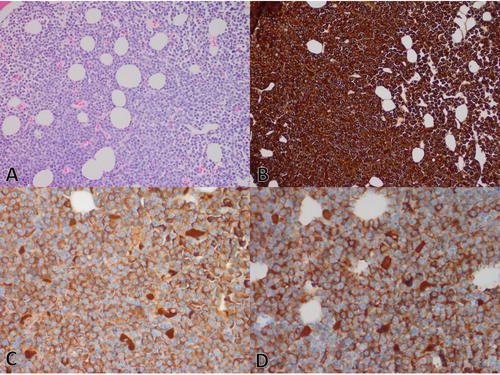
A 77-year-old white man was found to have anemia, hemoglobin 90 g/L, MCV 100 fL, neutrophils 1.7 × 109/L and platelets 125 x109/L during evaluation for anginal chest pain. Physical examination revealed normal S1, soft S2, apical grade 2/6 holosystolic murmur, decreased breath sounds in the right lung base and a distended abdomen. No lacrimal or salivary gland swelling was present. Bloodwork did not reveal eosinophilia (eosinophils 0.0 giga/L); random urine albumin/creatinine ratio was elevated at 29.7 (normal <2.0). Echocardiogram revealed moderate-to-severe mitral regurgitation from a ruptured posterior mitral valve leaflet cord. SPEP revealed a 48 g/L IgG lambda monoclonal band running in the beta-1 region, a monoclonal-free lambda light chain band in the beta-2 region, and polyclonal decrease in other immunoglobulins (). In retrospect, the patient had a SPEP two years prior demonstrating a 15 g/L IgG lambda monoclonal band in the beta-1 region. A small monoclonal IgG lambda in the beta-1 region and large monoclonal-free lambda light chain band in the beta-2 region were also noted in the urine protein electrophoresis. Serum lambda-free light chain concentration 5460 mg/L, serum kappa-free light chain 15.1 mg/L, with a lambda/kappa ratio of 361.6. IgG subclassification by liquid chromatography-tandem mass spectrometry showed predominance of the IgG4 subclass at 36.8 g/L [Citation13]. Repeat bloodwork revealed deteriorating renal function (serum creatinine of 429 µmol/L) and hypercalcemia (2.78 mmol/L), so the patient was admitted for urgent evaluation and management. Additional blood tests showed moderately prolonged activated partial thromboplastin time (APTT 52 s, reference: 25–38 s), and thrombin time (36 sec, reference: <25 s). APTT mixing studies showed correction of prolonged APTT on immediate mixing suggestive of interference from the paraprotein. Beta-2 microglobulin was markedly elevated at 45.2 mg/L, and interference from the paraprotein was suspected. A subsequent serum sample was analyzed for beta-2 microglobulin pre- and post-depletion of immunoglobulins. Immunoglobulin-depletion was performed by size exclusion using 100 K centrifugal filters (noting that the molecular weight of beta-2 microglobulin is ∼11 kDa and IgG is ∼150 kDa) and verified by SPEP, whereby the beta-2 microglobulin concentration was within the reference interval (<2.2 mg/L) in the immunoglobulin-depleted sample. Skeletal survey did not reveal any lytic bone lesions. Abdominal ultrasound and CT scan revealed only mild to moderate ascites with normal liver and spleen. Bone marrow studies revealed extensive infiltration with lambda-clonal plasma cell myeloma comprising between 60% and 80% of the cellular marrow without amyloid deposition (). The plasma cells have variable morphology, including intermediate maturity forms and less than 2% plasmablasts, consistent with intermediate myeloma [Citation18,Citation19]. They expressed CD38 and CD138, with aberrant expression of CD56 and CD117 and loss of CD19 and CD27. The plasma cells stained positive for IgG and IgG4 by immunohistochemistry. There was no evidence of storiform fibrosis or obliterative phlebitis. No evidence of 17p deletion or IGH translocation was noted by FISH. Full karyotype was not performed. The patient was diagnosed with IgG4 plasma cell myeloma (R-ISS Stage II) and started on weekly cyclophosphamide, dexamethasone and bortezomib. Subsequent laboratory results after three cycles of chemotherapy are summarized in .
Figure 2. Representative photomicrographs of marrow trephine biopsy in Case 2: (A) H&E showing subtotal replacement of the cellular marrow by plasma cells (200×). (B) Immunohistochemistry for Lambda showing lambda-restriction (200×). (C) Immunohistochemistry for IgG and (D) IgG4 showing the majority of plasma cells staining positive for both IgG and IgG4 (400×). On-slide negative and positive controls showed appropriate staining pattern (images not shown).
Discussion
IgG4 myeloma is a rare subtype of IgG plasma cell myeloma; in historical studies, its prevalence is thought to parallel the normal distribution of IgG4 subclass among healthy subjects (3-6% of IgG) and patients with plasma cell neoplasms (2-8% of IgG-positive cases) [Citation20,Citation21]. Neither of the two patients in this report had clinical or radiologic features of IgG4-related disease such as autoimmune pancreatitis, sialadenitis, orbital pseudotumor, retroperitoneal fibrosis or tubulointerstitial nephritis, despite presenting with very high levels of monoclonal IgG4. Both patients had a partial response to standard first-line myeloma therapy at the time of publication. In the second patient, the monoclonal band was present for at least 2 years before diagnosis of myeloma, presumably in the form of IgG4 monoclonal gammopathy of undetermined significance (MGUS). These cases suggest that elevated monoclonal serum IgG4 in isolation is not pathogenic for IgG4-related disease.
The precise role of IgG4 in the pathogenesis of IgG4-RD is an area of active investigation. Although the elevated serum IgG4 in IgG4-RD was initially suspected to be a reactive phenomenon, recent studies have highlighted IgG4-specific antibodies to antigens such as galectin 3 and Annexin A11 as contributors to raised serum IgG4 and IgE levels [Citation22,Citation23]. Moreover, the presence of ≥2 IgG4-specific antibodies against prohibitin, annexin A11, and laminin 511-E8 is associated with markers of increased disease severity such as higher serum IgG subclass levels, hypocomplementemia, and increased visceral organ involvement [Citation24]. Further studies exploring potential overlap in the role of B cells in both IgG4 myeloma and IgG4-RD are warranted, as IgG4+ plasma cells from IgG4-RD and myeloma patients express LOXL2, an enzyme implicated in tissue and bone marrow fibrosis [Citation25].
Of note, measurement of IgG subclasses in our center is done by a novel mass spectrometry assay. A major advantage of this assay over traditional nephelometric measurement of IgG subclasses, which is done in most other centers, is the avoidance of spurious elevation in IgG2 [Citation17,Citation26]. Had the IgG subclasses in this report been measured by nephelometry, it would have been difficult to ascertain whether these two cases represented IgG2 or IgG4 myeloma or a combination thereof. Another interesting observation to report is the markedly and spuriously elevated level of B2-microglobulin of 45.2 mg/L due to interference from the monoclonal IgG4 protein in Case 2. Whether this interference is simply due to the known interference that has rarely been reported with monoclonal proteins or is specific to IgG4 monoclonal proteins requires further study.
Since the advent of IgG4-RD, there have been few reports of IgG4 myeloma in the literature. One retrospective study of 158 bone marrow biopsies from patients with IgG myeloma identified six cases (4%) that were IgG4 subtype on immunohistochemical staining [Citation14]. Four of the six cases were men with a mean age of 64 years (range 53–87). All of these patients had elevated serum paraprotein (mean 24 g/L, range 5–42 g/L). In their report, the authors noted that three cases had plasmablastic morphology and two had necrotizing fasciitis. None of these six cases had evidence of IgG4-RD, although the authors did not report the actual serum IgG4 concentration in this case series. One case report described a case of kappa-restricted IgG4-positive myeloma with IgG4 levels of 48.8 g/L but did not provide any clinical or laboratory information about possible features of IgG4-RD [Citation16]. Kato et al. reported another case of kappa-restricted myeloma with increased monoclonal IgG4 (15.6 g/L) complicated by pancreatic ductal adenocarcinoma (initially misdiagnosed as localized autoimmune pancreatitis) [Citation15]. Only a few of these reports provided detailed information about response to treatment. One very recent study, published while this present paper was under review, describes 3 patients with IgG4 myeloma out of 80 patients with IgG myeloma from a Japanese cohort [Citation27]. Larger studies of IgG myeloma with subclassification of the serum paraproteins and plasma cell immunohistochemistry may provide insight into whether there are differences in the clinical presentation and prognosis for IgG1, IgG2, IgG3 and IgG4 myeloma.
Conclusion
Symptoms and clinical manifestations of IgG4 plasma cell myeloma appear to be similar with typical IgG plasma cell myeloma. Despite markedly elevated concentrations of serum IgG4 in the two cases described, clinical, radiographic and histological evidence of IgG4-RD were not observed. This implies that increased monoclonal serum IgG4 is not the primary etiologic mediator in IgG4-RD.
Ethical approval
Ethics review was waived as per our institutional guidelines for reports of two patients or less.
Acknowledgements
This work was supported by the Hal Kettleson Hematology Research Fund and the American Society of Hematology Visitor Training Program. The authors thank Dr Morris Pudek for his expert assistance with laboratory aspects of the study. D. G., K. M., M. D., A. F., G. V., A. M., M. C., K. S. and L. C. contributed to data collection, writing and finalizing the manuscript. D. G. and L. C. collected and analyzed the clinical data. M. D. M. conducting the experiments confirming IgG4 monoclonal with β2-microglobulin concentrations. Both patients consented to publication of their cases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Additional information
Funding
References
- Masaki Y, Shimizu H, Sato Nakamura T, et al. IgG4-related disease: diagnostic methods and therapeutic strategies in Japan. J Clin Exp Hematop. 2014;54(2):95–101. doi: 10.3960/jslrt.54.95
- Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet. 2015;385(9976):1460–1471. doi: 10.1016/S0140-6736(14)60720-0
- Bledsoe JR, Della-Torre E, Rovati L, et al. IgG4-related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018;126(6):459–476. doi: 10.1111/apm.12845
- Chen LYC, Mattman A, Seidman MA, et al. IgG4-related disease: what a hematologist needs to know. Haematologica. 2019;104(3):444–455. doi: 10.3324/haematol.2018.205526
- Qi R, Chen LYC, Park S, et al. Utility of serum IgG4 levels in a multiethnic population. Am J Med Sci. 2018;355(1):61–66. doi: 10.1016/j.amjms.2017.08.014
- Varghese JL, Fung AWS, Mattman A, et al. Clinical utility of serum IgG4 measurement. Clin Chim Acta. 2020;506:228–235. doi: 10.1016/j.cca.2020.04.001
- Zhao EJ, Carruthers MN, Li CH, et al. Conditions associated with polyclonal hypergammaglobulinemia in the IgG4-related disease era: a retrospective study from a hematology tertiary care center. Haematologica. 2020;105(3):e121–e123. doi: 10.3324/haematol.2019.219725
- Moller D, Tan J, Gauiran DTV, et al. Causes of hypereosinophilia in 100 consecutive patients. Eur J Haematol. 2020;105(3):292–301. doi: 10.1111/ejh.13437
- Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. doi: 10.1038/modpathol.2012.72
- Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 2020;79(1):77–87. doi: 10.1136/annrheumdis-2019-216561
- Kunkel HG, Fahey JL, Franklin EC, et al. Notation for human immunogobulin subclasses. Bull World Health Organ. 1966;35(6):953.
- Terry WD, Fahey JL. Subclasses of human gamma-2-globulin based on differences in the heavy polypeptide chains. Science. 1964;146(3642):400–401. doi: 10.1126/science.146.3642.400
- Grey HM, Kunkel HG. H chain subgroups of myeloma proteins and normal 7s gamma-globulin. J Exp Med. 1964;120:253–266. doi: 10.1084/jem.120.2.253
- Geyer JT, Niesvizky R, Jayabalan DS, et al. IgG4 plasma cell myeloma: new insights into the pathogenesis of IgG4-related disease. Mod Pathol. 2014;27(3):375–381. doi: 10.1038/modpathol.2013.159
- Kato S, Kuwatani M, Kawakubo K, et al. Hepatobiliary and pancreatic: pancreatic cancer with elevated serum IgG4 level due to multiple myeloma mimicking localized autoimmune pancreatitis. J Gastroenterol Hepatol. 2018;33(7):1310. doi: 10.1111/jgh.14088
- Zhu J, Wu B. Immunoglobulin G4-positive plasma cell myeloma. Blood. 2015;126(19):2254. doi: 10.1182/blood-2015-08-663492
- van der Gugten G, DeMarco ML, Chen LYC, et al. Resolution of spurious immunonephelometric IgG subclass measurement discrepancies by LC-MS/MS. Clin Chem. 2018;64(4):735–742. doi: 10.1373/clinchem.2017.282319
- Greipp PR, Raymond NM, Kyle RA, et al. Multiple myeloma: significance of plasmablastic subtype in morphological classification. Blood. 1985;65(2):305–310. doi: 10.1182/blood.V65.2.305.305
- Goasguen JE, Zandecki M, Mathiot C, et al. Mature plasma cells as indicator of better prognosis in multiple myeloma. New methodology for the assessment of plasma cell morphology. Leuk Res. 1999;23(12):1133–1140. doi: 10.1016/S0145-2126(99)00132-0
- Papadea C, Reimer CB, Check IJ. Igg subclass distribution in patients with multiple myeloma or with monoclonal gammopathy of undetermined significance. Ann Clin Lab Sci. 1989;19(1):27–37. doi: 10.3109/10408368909106589
- Aucouturier P, Preud'Homme JL. Subclass distribution of human myeloma proteins as determined with monoclonal antibodies. Immunol Lett. 1987;16(1):55–57. doi: 10.1016/0165-2478(87)90061-7
- Hubers LM, Vos H, Schuurman AR, et al. Annexin A11 is targeted by IgG4 and IgG1 autoantibodies in IgG4-related disease. Gut. 2018;67(4):728–735.
- Perugino CA, AlSalem SB, Mattoo H, et al. Identification of galectin-3 as an autoantigen in patients with IgG4-related disease. J Allergy Clin Immunol. 2019;143(2):736–745.e6. doi: 10.1016/j.jaci.2018.05.011
- Liu H, Perugino CA, Ghebremichael M, et al. Disease severity linked to increase in autoantibody diversity in IgG4-related disease. Arthritis Rheumatol. 2020;72(4):687–693. doi: 10.1002/art.41140
- Della-Torre E, Rigamonti E, Perugino C, et al. B lymphocytes directly contribute to tissue fibrosis in patients with IgG4-related disease. J Allergy Clin Immunol. 2020;145(3):968–981.e914. doi: 10.1016/j.jaci.2019.07.004
- Mattman A, Chen LYC, van der Gugten G, et al. In IgG4 related disease, elevated IgG2 is an artifact not a biomarker. Semin Arthritis Rheum. 2020;50(2):e8. doi: 10.1016/j.semarthrit.2019.08.002
- Ito A, Yamauchi T, Nakano A, et al. Igg4 plasma cell myeloma: clinicopathological characteristics and diagnosis. Pathol Int. 2020;70(8):551–556. doi: 10.1111/pin.12968
