ABSTRACT
Hemophagocytic lymphohistiocytosis (HLH) is an immune-mediated disorder caused by uncontrolled inflammatory responses and the activation of T lymphocytes. This life-threatening disease, characterized by fever, cytopenia and hepatosplenomegaly, is extremely rare during pregnancy with high mortality. Despite the improvement of treatment regimen in recent years, HLH is still a great challenge for clinicians. Here, we described a 26-year-old woman who admitted to our hospital at her first pregnancy with pyrexia. Her condition continued to deteriorate after receiving broad-spectrum antimicrobials, presenting with fever, pancytopenia, hepatosplenomegaly, ferritin ≥ 500 μg/L, hemophagocytosis and low NK-cell activity. HLH was eventually diagnosed by clinical manifestation and laboratory examination results. Then the patient recovered well after treatment with etoposide combined with ruxolitinib therapy and underwent successful induced-labor operation. Additionally, we summarized similar cases from the literature to improve the management of HLH during pregnancy. In conclusion, this study highlights the challenges and difficulties in the diagnosis and management of patients with HLH during pregnancy. Moreover, this is the first case report of etoposide combined with ruxolitinib in the treatment of patients with refractory secondary HLH during pregnancy.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a severe clinical syndrome characterized by a dysregulated hyperinflammatory immune response. It results in an uncontrolled proliferation, and activation of lymphocytes and macrophages, followed by secreting large amounts of cytokines called cytokine storm [Citation1]. The accumulation of activated macrophages can lead to the engulfing of erythrocytes, leukocytes, platelets and their precursor cells [Citation2].
HLH can be categorized as either primary or secondary. Primary HLH is mainly observed as familial HLH where patients have autosomal recessive mutations and can also present as inherited immunodeficiency syndromes [Citation3]. Secondary HLH can be triggered by various causes, including infections, hematological malignancies and autoimmune diseases in adults. Here, we reported a case of a patient with second HLH during pregnancy in the aspects of clinical symptoms, diagnosis and salvage therapy process. Relevant cases that published on Pubmed in the past 5 years were reviewed to summarize the clinical and laboratory parameters of HLH, as well as the outcome of the patients ().
Table 1. Characteristics of 25 cases of HLH during pregnancy.
Case report
A 26-year-old woman in her first pregnancy presented to the emergency department with fever. A pericoronitis abscess in her wisdom teeth was removed 25 days ago. She was first admitted to a local hospital and was considered for sepsis. Despite the early treatment with antibiotics, she had persistent fever and slowly developed into cytopenia, and bone marrow biopsy revealed 1% hemophagocytes. Then she was transferred to our hospital. Physical examination revealed pale face, body temperature (T) 39.2°C, heat rate 102 beats/min and blood pressure 107/67 mmHg. The cardiopulmonary and abdominal examinations yielded unremarkable results, and there was no evidence of lymphadenopathy. She denied cough, abdominal pain, diarrhea, abnormal vaginal secretions, alopecia, rash and joint pain. There was no history of recent travels. She was allergic to sulfonamides, and hypothyroidism was observed at fifth week of pregnancy.
Hemogram showed hemoglobin (Hb) 75 g/L (normal, 115–150 g/L), mean corpuscular volume (MCV) 83.1 fl (normal, 82–95 fl), white blood cell count (WBC) 5.3 × 109/L (normal, 4–10/L), neutrophil (NEUT) 3.95 × 109/L (normal, 1.80–6.40/L) and platelet (PLT) count 41 × 109/L (normal, 100–300/L). Peripheral blood film demonstrated 0.23% of neutrophilic lobulated granulocytes, the occasional red blood cell fragments and 0.01 of middle and late immature granulocytes. In addition, other relevant laboratory data were as follows: serum procalcitonin (PCT) 1.61 ng/mL (normal, 0.00–0.05 ng/mL), C-reactive protein (CRP) 131.96 mg/L (normal 0.00–10.00 mg/L). Meanwhile, serum ferritin, lactate dehydrogenase (LDH), and triglyceride concentrations were 1420 ug/L (normal, 114–240 ug/L), >1500 U/L (normal, 16.4–323.0 U/L), 1.99 mmol/L (normal, 0.33–1.70 mmol/L), respectively. Results of thyroid function test were normal. The color Doppler ultrasound of gynecology revealed intrauterine pregnancy, fetal survival and normal umbilical blood flow. Extensive infectious tests, including blood culture, respiratory pathogens, fungal glucan detection, aspergillus cryptococci antigen detection, EBV and CMV antibodies, HIV, tuberculin, hepatitis virus series, were negative. The report of chest and abdomen computerized tomography (CT) scan showed an abnormal enlargement of spleen (about 8 costal units), the presentation of many small lymph nodes in retroperitoneal and splenic hilus. At this point, the overall clinical picture highly suggested of HLH. Then, 10 mg/day of dexamethasone and 10 g/day of intravenous immunoglobulin (IVIG) were administrated to improve patient’s physical condition.
The detection of autoimmune markers included antinuclear antibody, vasculitis, direct/indirect coombs test and pathological anticoagulant without abnormality. Echocardiography indicated negative infectious endocarditis. On day 6, a bone marrow biopsy showed the signs of HLH with the evidence of hemophagocytes (A).
Figure 1. Hematoxylin and eosin stain (HE) of bone marrow aspirate smear (left anterior superior iliac spine) showed the evidence of hemophagocytosis, Macrophage engulfing erythrocytes, platelets, and neutrophils; original magnification ×400 (A). Bone marrow pathology: bone marrow hyperplasia is generally normal, three lines of hematopoietic cells can be seen, with lobulated megakaryocytes and no tumor cells, original magnification ×40 (B).
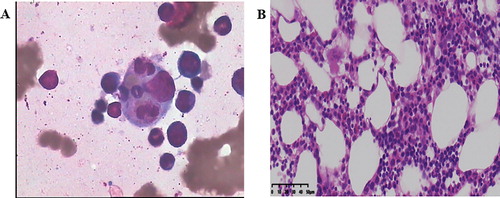
The patient declared no family history of HLH. Further investigations for the underlying cause of HLH were conducted. The PET-CT showed hepatosplenomegaly and active metabolism of spleen. Both bone marrow metabolism of pelvis and the possibility of reactivity change remained high, whereas the lymphoma was not identified. The results of peripheral blood tumor cells (CTC) were negative. An abnormal presentation of sCD25 (soluble IL-2 receptor) was seen as 3169 U/mL (normal, 223–710 U/mL), while NK-cell activity was only 2.5% (normal, > 2.6%). On day 14, patient was transferred to the hematology department. The bone marrow biopsies of left and right posterior superior iliac spine (PSIS), and right anterior superior iliac spine (ASIS) were performed to further exclude focal lymphoma and detect its underlying cause of HLH. The results of pathological examination showed no hematological diseases, and pathological results of bone marrow biopsy of left ASIS suggested the presentation of three-line hematopoietic cells, normal proportion of granulocyte/red and no observation of tumor cells (B). No HLH-related mutations were detected (Wuhan kangshengda, specimen No. s190922033). Consequently, the patients met seven out of eight clinical criteria for HLH (), as shown by fever (T > 38°C), pancytopenia, hepatosplenomegaly, ferritin ≥ 500 μg/L, observed hemophagocytosis on the bone marrow biopsy, low NK-cell activity and sCD25 ≥ 2400 U/mL. Finally, she was diagnosed with secondary HLH.
Table 2. The diagnostic guidelines for HLH updated in 2004.
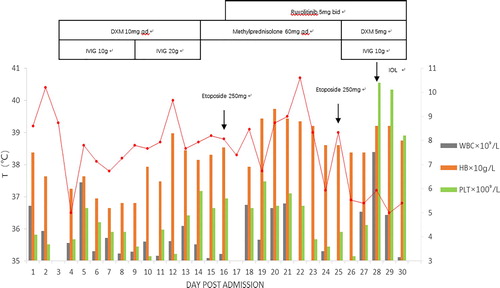
At first, HLH-directed therapy (methylprednisolone 60 mg/day and IVIG 20 g/day) was adopted. However, the patient continued to have daily fever and needed multiple blood product support because of severe pancytopenia. To improve the patient's physical condition, 250 mg of etoposide plus 5 mg/m2 of ruxolitinib (dose adjusted for pregnancy, standard therapy 15–20 mg twice daily) were applied on day 16. Then, the patient became afebrile after the new drug treatment. After 72 h, the blood tests revealed HB 89 g/L, WBC 3.88 × 109/L, NEUT 3.95 × 109/L and PTL 63 × 109/L. On day 25 of hospitalization, the patient received a second dose of etoposide. Owing to the use of multiple drugs during pregnancy, the patient decided to terminate her pregnancy. A drug induced labor operation was successfully completed under the close monitoring. The pathological results of placenta and umbilical cord were normal. Patient was then discharged on day 30.
On day 4 after discharge, the patient was re-admitted to the hospital for the third chemotherapy of etoposide. The laboratory results demonstrated that the level of sCD25 was reduced from 3169 to 460 U/mL, the activity of NK cell was elevated to 4.3%, and content of HB and PLT were return to the normal range. The patient recovered well. The therapeutic schedule, body temperature, and hemogram of this patient were showed in .
Discussion and literature review
This case illustrated the challenge of diagnosis and salvage therapy of secondary HLH in a pregnant lady. The pregnant woman of HLH often present with fever, cytopenia, hepatomegaly, splenomegaly and peripheral lymphadenopathy; some subjects even have jaundice, coagulation dysfunction, skin abnormality (rash, erythema and purpura) and central nervous system involvement (encephalopathy, meningitis and epilepsy) [Citation2]. It is worth noting that fever and cytopenia (anemia and thrombocytopenia) are occurred in most HLH patients [Citation4]. None of these features are specific for HLH, which made it harder to differentiate HLH from other diseases, such as sepsis and autoimmune diseases.
Due to the rarity of HLH and similar clinical presentations to other diseases, HLH is easily missed during pregnancy. Studies have shown that the cytokines are released by immune cells, particularly T-helper (Th) lymphocytes. The secretion of proinflammatory cytokines by Th1 cells promotes the cell-mediated immune responses, while the cytokines induced by Th2 cells can trigger the production of antibody [Citation5]. During pregnancy, Th lymphocytes typically shift from Th1 to Th2 dominance because of the immune adaptation to the genetically foreign fetus and antigenicity. This allows the reduction of cell-mediated immunity (CMI) of Th1 cells increasing the susceptibility to virus infection [Citation5]. Teng et al. have reported that immature placenta releases trophoblast debris, including syncytiotrophoblast components, soluble RNA and DNA of fetal origin and cytotrophoblast cells, into the maternal circulation. A profound systemic inflammatory response induced by this feto-maternal trafficking may mimic the pregnancy-induced HLH [Citation6]. Many current evidences support that the patient in this study had pregnancy-related HLH.
The diagnosis of HLH mainly relies on the coexistence of clinical manifestations and laboratory results. A diagnostic criteria guideline of HLH updated in 2004 has been widely used, and HLH is diagnosed if 5 out of 8 criteria are met () [Citation7]. As shown in , changes in blood count are common. All the 11 patients had abnormal hemogram, and there were 4 patients with Pancytopenia [Citation8–10], 6 patients had anemia and thrombocytopenia [Citation4,Citation11–15]. Only one patient had thrombocytopenia [Citation10]. It is worth noting that both ferritin level and serum sIL-2r (sCD25/sIL-2r) are diagnostic indicators of HLH. Peak ferritin levels often >10,000 g/L had 90% sensitivity and 96% specificity for HLH [Citation16]. The optimal cut-off of sCD25 is 2515 U/mL (sensitivity 100% and specificity 72.5%) [Citation17]. The serum ferritin, sCD25 of the patient in this case were significantly elevated as 1420 u/L, 3169 U/mL respectively. Recently, Fardet et al. have developed and validated a diagnostic score named HScore online, which can be used to estimate the risk of reaction HLH on an individual (http://saintantoine.aphp.fr/score/) [Citation18].
Finding the evidence of hemophagocytes on bone marrow biopsy and other organ tissues (such as liver, spleen, and lymph nodes) are recommended for the diagnosis of HLH. One recent study showed that approximately 30% patients with HLH did not exhibit any hemophagocytic features on their biopsies [Citation19]. Sometimes repeat biopsy is necessary. Among the 11 patients we reviewed, 8 patients found hemophagocytes in the first bone marrow biopsy [Citation4,Citation9–14] and 1 patient in the second biopsy [Citation15]. Only one patient found hemophagocytes in the first liver biopsy [Citation10], and the other patient found hemophagocytes at repeated liver biopsy [Citation14]. The biopsy rate was 100%, and the positive rate was close to 91%. Although biopsy is an invasive procedure that should be avoided during pregnancy, it is of great significance for the detection of hemophagocytes. Hence, we believed that biopsy on bone marrow or other organ tissues should be considered and repeated if a patient is suspected of HLH during pregnancy.
The treatment of secondary HLH is intended to suppress life-threatening inflammatory process and deal with underlying causes [Citation4]. Major drugs, such as steroids, IVIG, cyclosporine A and etoposide, can be used to reduce the hyperinflammation and hyperinflammatory responses [Citation13]. A study conducted by Rupali Das has confirmed that treatment with JAK1/2 inhibitor ruxolitinib can significantly reduce the clinical and laboratory manifestations in murine models of HLH. He suggested that ruxolitinib targeted inflammation responses in a different manner from steroids and etoposide, so ruxolitinib might synergize with other agents to treat HLH [Citation20]. A pilot study of ruxolitinib in adults with secondary HLH has revealed that all patients achieved a good response after ruxolitinib treatment, allowing the transfusion independence, corticosteroids discontinuation, and hospital discharge [Citation21]. Besides, two recent cases have clarified that as a first line treatment for refractory secondary HLH, ruxolitinib can rapidly improve the relative laboratory outcomes, which supports further investigation of cytokine-directed approaches in treating secondary HLH [Citation22,Citation23].
Moreover, treatment guidelines for HLH during pregnancy have not been established elsewhere due to the side effects on fetus. Now, the safest treatment is corticosteroids and IVIG with treatment of the underlying cause. Among the 11 related cases summarized in , only three patients were effective with steroids or IVIG regimen alone [Citation8,Citation9,Citation14]. Wang et al. have reported that etoposide can be bravely considered as an alternative drug especially for pregnancy-related HLH who had no response to corticosteroids/IVIG therapy. But, suitable dosages and toxic and side effects require further clinical observation [Citation24]. Among the 11 patients, six were treated with etoposide on the basis of steroids or IVIG regimen, and only two patients survived [Citation10,Citation15]. Obviously, some patients even had no effective responses to the traditional treatment regimen. Therefore, finding a target and effective drug therapy for HLH during pregnancy remains a huge challenge in clinical settings.
Ruxolitinib is a safe and effective salvage therapy to improve the inflammatory status for refractory HLH; whereas its remission depth may not be sufficient. A prospective multicenter large-scale clinical trial is underway to validate the efficacy of doxorubicin–etoposide–methylprednisolone–ruxolitinib (DEP-Ru) regimen on refractory/relapsed HLH (ClinicalTrails.gov Identifier: NCT03533790) [Citation25]. In this case report, the patient had persistent fever, severe pancytopenia, and deterious physical conditions after giving aggressive traditional treatment regimen. Nevertheless, after administration with etoposide plus ruxolitinib drug therapy, she became afebrile along with improved laboratory values, and the induced-labor operation was allowed to perform afterwards. It is clear that the combination of etoposide and ruxolitinib has created a suitable condition for induced-labor operation. To our knowledge, this is the first case in which etoposide combined with ruxolitinib used as the first-line treatment for salvage refractory HLH during pregnancy. More clinical practices are still needed to support the significance of this combined treatment regime.
In conclusion, although the treatment regimen has been improved over the years, HLH patients during pregnancy remain a challenge to clinicians. There are only 6 out of 11 patients (54.5%) survived in the end (). Therefore, the mortality rate of this disease is high, and clinicians must have a high sense of vigilance. In addition to the regular inspection items, we need to check the ferritin level, sCD25 and biopsy as soon as possible to identify the diagnosis. This study highlights the clinical and laboratory parameters of HLH in pregnant woman and summarizes the experience of previous literatures regarding the diagnosis and management of HLH. For patients with refractory secondary HLH during pregnancy, etoposide combined with ruxolitinib may be an effective treatment to improve the symptoms and create optimal physical conditions of patients for follow-up treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brisse E, Wouters CH, Matthys P. Hemophagocytic lymphohistiocytosis (HLH): a heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 2015;26(3):263–280.
- Creput C, Galicier L, Buyse S, et al. Understanding organ dysfunction in hemophagocytic lymphohistiocytosis. Intensive Care Med. 2008;34(7):1177–1187.
- Hayden A, Park S, Giustini D, et al. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: a systematic scoping review. Blood Rev. 2016;30(6):411–420.
- Tumian NR, Wong CL. Pregnancy-related hemophagocytic lymphohistiocytosis associated with cytomegalovirus infection: a diagnostic and therapeutic challenge. Taiwan J Obstet Gynecol. 2015;54(4):432–437.
- Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283(5406):1277–1278.
- Teng CL, Hwang G-Y, Lee B-J, et al. Pregnancy-induced hemophagocytic lymphohistiocytosis combined with autoimmune hemolytic anemia. J Chin Med Assoc. 2009;72(3):156–159.
- La Rosee P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477.
- Samra B, Yasmin M, Arnaout S, et al. Idiopathic hemophagocytic lymphohistiocytosis during pregnancy treated with steroids. Hematol Rep. 2015;7(3):6100.
- Rousselin A, Alavi Z, Le Moigne E, et al. Hemophagocytic syndrome in pregnancy: case report, diagnosis, treatment, and prognosis. Clin Case Rep. 2017;5(11):1756–1764.
- Parrott J, Shilling A, Male HJ, et al. Hemophagocytic lymphohistiocytosis in pregnancy: a case series and review of the current literature. Case Rep Obstet Gynecol. 2019;2019:9695367.
- Giard JM, Decker KA, Lai JC, et al. Acute liver failure secondary to hemophagocytic lymphohistiocytosis during pregnancy. ACG Case Rep J. 2016;3(4):e162.
- Kerley RN, Kelly RM, Cahill MR, et al. Haemophagocytic lymphohistiocytosis presenting as HELLP syndrome: a diagnostic and therapeutic challenge. BMJ Case Rep. 2017;2017:bcr2017219516), doi: 10.1136/bcr-2017-219516.
- He MZ, Jia J, Zhang JY, et al. Pregnancy-associated hemophagocytic lymphohistiocytosis secondary to NK/T cells lymphoma A case report and literature review. Medicine (Baltimore). 2017;96(47):6.
- Yildiz H, Vandercam B, Thissen X, et al. Hepatitis during pregnancy: a case of hemophagocytic lymphohistiocytosis. Clin Res Hepatol Gastroenterol. 2018;42(3):e49–e55.
- Cheng J, Niu J, Wang Y, et al. Hemophagocytic lymphohistiocytosis in pregnancy: a case report and review of the literature. J Obstet Gynaecol. 2020;40(2):153–159. doi: 10.1080/01443615.2019.1601168.Epub 2019 Jun 19
- Larroche C. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine. 2012;79(4):356–361.
- Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529–2534.
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620.
- Riviere S, Galicier L, Coppo P, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127(11):1118–1125.
- Das R, Guan P, Sprague L, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666–1675.
- Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6(12):e630–e6e7.
- Slostad J, Hoversten P, Haddox CL, et al. Ruxolitinib as first-line treatment in secondary hemophagocytic lymphohistiocytosis: a single patient experience. Am J Hematol. 2018;93(2):E47–EE9.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Song Y, Wang Z, Hao Z, et al. Requirement for etoposide in the treatment of pregnancy related hemophagocytic lymphohistiocytosis: a multicenter retrospective study. Orphanet J Rare Dis. 2019;14(1):50.
- Wang J, Wang Y, Wu L, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020 May;105(5):e210–e212. doi: 10.3324/haematol.2019.222471.Epub 2019 Sep 12