ABSTRACT
Objectives
To evaluate the efficacy and adverse effects of venetoclax(VEN) in combination with hypomethylating agents(HMAs) in acute myeloid leukemia(AML) or myelodysplastic syndrome(MDS).
Methods
Clinical studies were identified from the Cochrane Library, PubMed, Embase, Google Scholar, and ClinicalTrials.gov. Overall complete remission (CR) and overall response rate (ORR) were used to evaluate the efficacy of VEN in combination with HMAs for AML/MDS, the incidence of the 4 most common grade 3–4 adverse events was used to evaluate safety.
Results
We identified 13 studies that included a total of 1059 patients. 7 cohort studies and 5 non-randomized controlled trials(NRCTs) were analyzed by random-effects model, and subgroup analyses showed the pooled overall CR rate of 62% (95% CI 57-67%, I2 = 3%) for the new-diagnosed(ND) AML group, 39% (95% CI 30-48%, I2 = 28%) for relapsed/refractory(R/R)-AML, and 61% (95% CI 50-71%, I2 = 25%) for MDS, respectively. There was only one randomized controlled trial(RCT) that showed a CR rate of 66.4% in the patients who received azacitidine(AZA) plus VEN. A total of 8 studies reported adverse events, with cytopenia and infection being the most common grade 3–4 adverse events.
Conclusions
The addition of VEN to HMAs may provide significant clinical benefit for AML/MDS patients, where response rates are better in MDS and ND-AML than in R/R-AML, but attention should be paid to the possible increased risk of febrile neutropenia.
Introduction
AML is a myeloid hematopoietic stem/progenitor cell malignancy characterized by abnormal proliferation of primitive cells in the bone marrow and peripheral blood, with a median age of 67 years [Citation1]. The prognosis of AML is relatively poor, with the reported median overall survival(OS) of only 9.2 months and 5-year OS of only 13.5% [Citation2]. At present, induction therapy is mainly based on the standard dose of cytarabine and anthracyclines, known as the 'DA3 + 7' protocol. Consolidation therapy is dominated by medium or high doses of cytarabine and allogeneic hematopoietic stem cell transplantation. The National Comprehensive Cancer Network (NCCN) recommends HMAs such as decitabine(DEC) and AZA as the treatment of choice for older AML patients, including unfavourable cytogenetics, poor molecular markers, a history of antecedent hematologic disorder, treatment-related AML or incorrect performance status. AZA and DEC also have been considered standard of care for MDS. However, the response rate of HMAs in AML/MDS patients remains undesirable.
The antiapoptotic protein B-cell leukemia/lymphoma-2(BCL-2) is overexpressed in hematologic malignancies and is associated with maintenance and survival of AML cells, treatment resistance and poor overall survival in AML patients. In addition, abnormal overexpression of BCL-2 has been found in patients with high-risk MDS. VEN is an oral, potent BCL-2 inhibitor that causes rapid tumour cell apoptosis by specifically inhibiting the Bcl-2 protein and activating the endogenous mitochondrial apoptotic pathway [Citation3]. Recent clinical data have shown that VEN in combination with HMAs has a CR/CR with imcomplete recovery of peripheral blood counts(CRi) rate of 67% in ND-AML patients over 65 years of age who are not candidates for intensive therapy [Citation4], and the Food and Drug Administration(FDA) granted accelerated approval to VEN in combination with HMAs for the treatment of ND-AML in adults who are aged 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. Related preclinical studies also have shown The addition of VEN to AZA may benefit patients with high-risk MDS [Citation5]. However, most of the published studies are generally small in scale, so evidence for these study results remains relatively limited.
The aim of this systematic review and meta-analysis was to identify all studies using the combination therapy of VEN and HMAs for the treatment of patients with AML/MDS and to pool their results to fully evaluate their efficacy and adverse events.
Methods
Data sources and searches
The present systematic review and meta-analysis was conducted and reported according to the standards of quality detailed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The present study was registered at the International Prospective Register of Systematic Reviews (registration number: CRD42020176315). Two investigators independently searched for studies published before August 2020 in PubMed, Cochrane Library, Embase, Google Scholar and ClinicalTrials.gov. In addition, we manually retrieved two journals, Blood and Leukemia, the references of the eligible articles were also reviewed. The keywords used in PubMed were ‘decitabine or dacogen’, ‘azacitidine or vidaza’, ‘hypomethylating agent or HMA’, ‘venetoclax or ABT-199 or GDC-0199’, ‘acute myeloid leukemia or AML’ and ‘Myelodysplastic Syndrome or MDS’. The detailed search strategy is available as supplementary data.
Inclusion and exclusion criteria
Inclusion criteria: (1) AML or MDS patients treated with VEN in combination with AZA or DEC at any dose and duration of therapy. (2) Original study was a prospective cohort study, retrospective cohort study, NRCT or RCT. (3) The primary outcomes were overall CR rate. The secondary outcomes were ORR, median OS and the rate of grade 3–4 adverse events including decreased white blood cell(WBC) count, thrombocytopenia, anemia and febrile neutropenia.
Exclusion criteria: (1) Study population other than AML or MDS. (2) Type of study was non-clinical, review, case report or meta-analysis. (3) Treatment regimen included drugs other than VEN in combination with AZA or DEC. (4) Study results were repeatedly reported in the literature. (5) Study did not include primary outcome measures.
The eligibility assessment was conducted independently by two researchers. If the eligibility of the study was assessed differently, the two investigators jointly reviewed the study and a consensus was eventually reached.
Definition of treatment response and outcomes
A CR was defined as the presence of all the following: a bone marrow blast count of < 5%; the absense of circulating blasts and blasts with Auer rods; the absence of an extremedullary disease; a hemoglobin level of ≥ 11 g/dL; an absolute neutrophil count of ≥ 1.0 × 109/L; and a platelet count of ≥ 100 × 109/L. CRi or Complete bone marrow remission(mCR) was defined as meeting the CR criteria. A partial remission was defined as a bone marrow blast count which declined by at least 50% relative to the pretherapy level but still remained above 5%[Citation6]. The overall CR rate was the proportion of patients who achieved CR, CRi or mCR after therapy; the ORR was defined as the proportion of patients who achieved CR, CRi, mCR, partial remission, morphologic leukemia free state(MLFS) or hematological improvement(HI) after therapy. grade 3–4 decreased WBC count, thrombocytopenia, anemia and febrile neutropenia were defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.
Statistical analysis
All data analysis was performed using R software version 3.6.3(R Foundation for Statistical Computing, Vienna, Austria). Two investigators used pre-designed data extraction table to extract data from each study. Random-effects model using PLOGIT transformations were used to combine the incidence and 95% confidence intervals for overall CR, ORR, and grade 3–4 adverse events. Heterogeneity between studies was assessed using Cochran's test and I2 statistics. The I2 values were classified as follows: 0–25% indicated insignificant heterogeneity; 26–50%, low heterogeneity; 51–75%, moderate heterogeneity; and > 75%, high heterogeneity[Citation7]. The quality of the included studies were assessed strictly according to the Newcastle-Ottawa Scale(NOS) and Cochrane risk of bias tool.
Results
Baseline patient characteristics
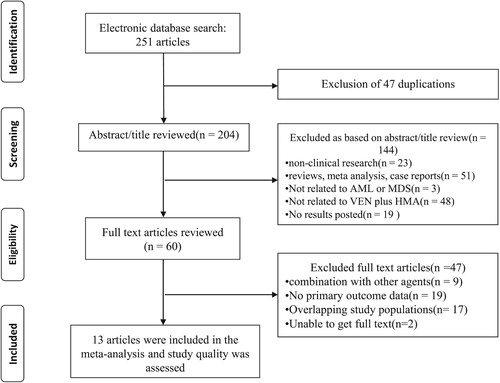
A total of 251 articles were eventually retrieved. After excluding 47 duplicate articles using Endnote X9 software, the remaining 204 articles were screened by reading the abstracts and full text, and ultimately 13 studies[Citation4, Citation8–19] (7 retrospective cohort studies, 5 NRCTs and one RCT) met the inclusion criteria and were included in the meta-analysis. The selection process was documented in a flow chart recommended by the PRISMA (). A total of 934 AML and 125 MDS patients, aged between 18 and 91 years, of whom more than 50% were male and elderly(>60 years). Most patients (>50%) were considered to have unfavourable-risk AML and MDS according to the genetic risk stratification used in all studies. The clinical characteristics of these patients were tabulated in and the study design and treatment regimen in .
Table 1. Baseline characteristics of included studies.
Table 2. The study design and treatment regimen used in the included studies.
The cohort studies and NRCTs were evaluated using the NOS, with scoring between 7 and 9 and considered 'high quality'; the RCT was evaluated using the Cochrane risk of bias tool, which was scored 5 and also considered 'high quality'. The detailed scores was tabulated in .
Table 3. The quality of the included studies assessed by NOS.
Efficacy
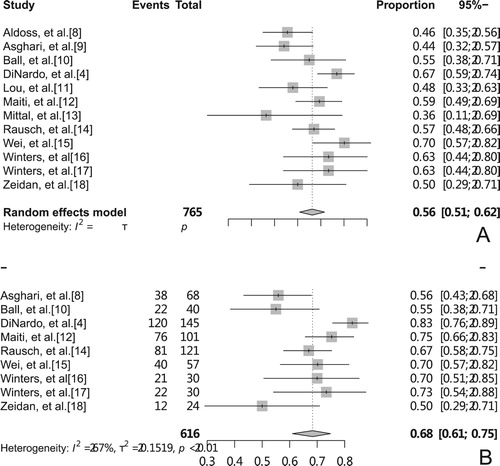
Seven cohort studies and 5 NRCTs were analyzed by random-effects model and the results showed pooled overall CR rate after treatment with VEN + HMA regimen was 56%(95% CI, 51-62%, I2 = 51%, (A)), while the pooled ORR was 68%(95% CI, 61–75%, I2 = 67%, (B)). Results showed moderate heterogeneity. A total of 7 studies reported median OS, and a descriptive systematic evaluation of median OS was observed in the range of 4.9–17.5 months.
The CR and median OS in VEN + AZA groups was 66.4% and 14.7 months, respectively, in the study by DiNardo et al. [Citation19].
Adverse evnets
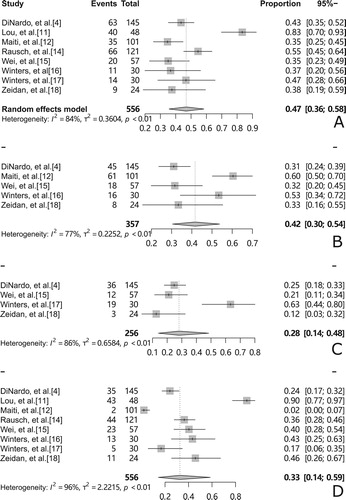
A total of 8 studies reported adverse events, with cytopenia and infection being the most common grade 3–4 adverse events, and adverse events were combined for 7 of these studies. The pooled rate of febrile neutropenia was 47%(95% CI, 36-58%, I2 = 84%, (A)). The pooled rate of grade 3–4 decreased WBC count was 42%(95% CI, 30-54%, I2 = 77%, (B)). The pooled rate of grade 3–4 anemia was 28%(95% CI, 14-48%, I2 = 86%, (C)). The pooled rate of grade 3–4 thrombocytopenia was 33%(95% CI, 14–58%, I2 = 96%, (D)).
Figure 3. Forest plots of the most common grade 3–4 adverse events (A): pooled febrile neutropenia rate (B): rate of grade 3–4 decreased WBC count (C): rate of grade 3–4 anemia (D): rate of grade 3–4 thrombocytopenia.
In the study by DiNardo et al. [Citation19], the incidences of grade 3–4 adverse events in patients who received VEN + AZA regimen were 42% of the patients with febrile neutropenia, 20% with decreased WBC count, 26% with anemia, 45% with thrombocytopenia, respectively.
Sensitivity analysis
Sensitivity analysis was performed by removing individual studies in turn and observing changes in the pooled rate, which showed that the pooled rates were all within the CI of the overall pooled rate, suggesting that the results were stable. The study by DiNardo et al. [Citation4] had the largest influence on the heterogeneity of the ORR rate. Removal of this study changed the ORR by 2% (from 68% to 66%) and led to a loss of heterogeneity (I2 = 38.6%), possibly related to the deletion of AML patients with favorable-risk cytogenetics from the study.
Subgroup analysis
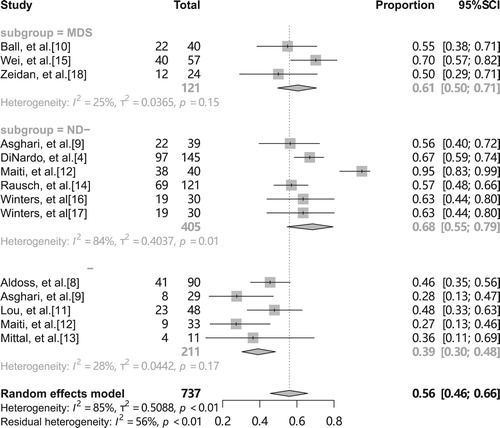
A recent meta-analysis [Citation20] showed that the CR/CRi rate of VEN in combination with HMAs or low-dose acytidine in patients with R/R-AML was 32.8%, compared with a significant difference of 67% in the clinical trial of DiNardo et al. [Citation4] in treatment-naive, elderly patients with AML. Thus we consider that heterogeneity of outcomes may also stem from different disease subtypes, and we divide the inclusion population into three subgroups according to disease type: ND-AML, R/R-AML and MDS. The results showed a pooled overall CR rate of 68% (95% CI 55-79%, I2 = 84%, ) for the ND-AML group, 39% (95% CI 30-48%, I2 = 28%, ) for R/R-AML, and 61% (95% CI 50-71%, I2 = 25%, ) for MDS. The results showed no significant heterogeneity between the remaining two groups except for the ND-AML group. It is evident from that the study by Maiti et al. [Citation12] had the greatest effect on the heterogeneity of the combined and overall CR rates, and deletion of this study changed the pooled overall CR rate by 6% (from 68% to 62%, 95% CI 57-67%) and made the heterogeneity disappear (I2 = 3%). We think that this may be due to the fact that the treatment regimen in this study was 10-day DEC with VEN rather than the standard 5-day DEC with VEN.
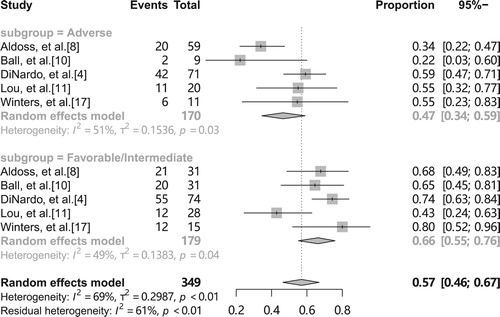
For different genetic risk stratification, we set up a subgroup analysis for both favorable/intermediate and adverse groups, which showed a pooled overall CR rate of 66% (95% CI 55-76%, I2 = 49%, ) for favorable/intermediate and 47% (95% CI 34-59%, I2 = 51%, ) for adverse, indicating a relatively low pooled overall CR rate in patients with adverse-risk AML/MDS, but the results is not stable due to moderate heterogeneity. The combined adverse event results were significantly heterogeneous, which we believe may have stemmed from differences in disease type, but the data required were insufficient to allow subgroup analysis.
Discussion
This meta-analysis included 13 studies with a total of 1059 patients and comprehensively evaluated the efficacy and adverse events of VEN in combination with HMAs in patients with AML/MDS. The combined results of 7 cohort studies and 5 NRCTs showed that the pooled overall CR and ORR rate were 56% and 68%, respectively, and the I2 obtained by the heterogeneity test were all between 51 and 70% with moderate heterogeneity. Sensitivity analysis perfomed by excluding inclusion studies in order, suggesting stable results. We consider that heterogeneity may stem from differences in age group, disease subtype, cytogenetic risk, intervention, etc. The subgroup analysis of different disease subtypes showed that the pooled overall CR rates of ND-AML and MDS groups were higher than those of R/R-AML groups, and there was no overlap in the confidence intervals, indicating that there was no difference between the subgroups and the results were stable. A subgroup analysis of different genetic risk stratification revealed overlapping confidence intervals for pooled overall CR rates between favorable/intermediate and adverse groups, suggesting that there are differences in results and that clinical trial redesign is needed to validate. In addition, we analyzed the adverse events and a total of 8 studies reported adverse events, with cytopenia and infection being the most common grade 3–4 adverse events. However, there was significant heterogeneity in the results of the analyses, possibly due to differences in disease type or treatment regimen, and there was insufficient data for further subgroup analysis.
A recent meta-analysis comparing HMA monotherapy with HMA plus Chemotherapy for Intermediate/High-Risk MDS or AML showed an ORR of only 42% for HMA monotherapy [Citation21], greatly lower than that of our study, indicating that adding VEN to HMAs resulted in a significant benefit for patients with AML or MDS; another meta-analysis evaluating adverse events with HMA monotherapy in AML or MDS patients showed 25% with febrile neutropenia [Citation22], significantly lower than our study, implies that the addition of VEN to HMAs may increase the incidence of febrile neutropenia.
The study by DiNardo et al. [Citation19] showed significantly longer OS (median OS: 14.7 months vs 9.6 months), a 34% lower risk of death (HR=0.66; 95% CI: 0.52-0.85; p<0.001) and more than double the rate of CR + CRi(66.4% vs 28.3%, p<0.001) in the VEN + AZA group compared with the AZA + placebo group. Among the grade 3–4 adverse events reported in this study, the incidence of febrile neutropenia was also significantly higher in the VEN + AZA regimen group than in the AZA + placebo group(42% vs 19%).
This study overcame the shortcomings of the previous study, based on a comprehensive literature search, evaluated RCT, NRCTs and cohort studies using appropriate criteria, meta-analysis of NRCT and cohort studies, qualitative analysis of RCT. Inevitably, there are some limitations. Firstly, we included only one RCT, so high quality meta-analysis of RCT could not be performed. Secondly, moderate heterogeneity was found in the data analysis. To find the source of the heterogeneity, a subgroup analysis was performed and the results suggest that differences in disease subtypes may be the main source of heterogeneity. Thirdly, OS was not explored in detail due to the varying length of follow-up. Lastly, due to insufficient data from the original study, we were unable to perform subgroup analyses for different ages, treatment regimens, adverse events, etc, and it is suggested that follow-up studies could set up subgroups to control for the above factors in order to obtain fuller evidence.
Conclusions
The addition of VEN to HMAs may provide significant clinical benefit for AML/MDS patients, where response rates are better in MDS and ND-AML than in R/R-AML, but attention should be paid to the possible increased risk of febrile neutropenia. However, there is still a need for RCT to comprehensively evaluate the efficacy and adverse effects of the VEN + HMAs regimen in patients with AML and MDS.
Ethics approval and consent to participate
The need for ethics approval by an institutional board review was waived as this study does not directly involve human subjects.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Notes
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; N, not reported; R/R, Relapsed/refractory; HR, high-risk; VEN, venetoclax; AZA, azacitidine; DEC, decitabine.
Abbreviations: IV, Intravenous; H, Subcutaneous.
⋆ and⋆⋆indicates that the eight quality test options were fullfiled in the studies. See more explanations at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
References
- Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7.
- Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome. Cancer. 2006;106(5):1090–1098.
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discovery. 2008;7(12):989–1000.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- Bogenberger JM, Kornblau SM, Pierceall WE, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014;28(8):1657–1665.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425.
- Higgins JPT, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557.
- Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019;94(10):E253–e255.
- Asghari H, Lee D, Deutsch YE, et al. Outcomes of patients with Relapsed or refractory acute myeloid leukemia Receiving hypomethylating agent and venetoclax. Blood. 2019;134(Suppl 1):1357–1357.
- Ball BJ, Famulare C, Stein EM, et al. Combined venetoclax and hypomethylating agent (HMA) therapy Induces high response rates in patients with myelodysplastic syndrome including patients Previously Failing HMA. Blood. 2019;134(Suppl 1):4241–4241.
- Lou Y, Shao L, Mao L, et al. Efficacy and predictive factors of venetoclax combined with azacitidine as salvage therapy in advanced acute myeloid leukemia patients: A multicenter retrospective study. Leuk Res. 2020;91:106317.
- Maiti A, DiNardo CD, Rausch CR, et al. Ten-Day decitabine with venetoclax (DEC10-VEN) in acute myeloid leukemia: Updated results of a phase II trial. Blood. 2019;134(Suppl 1):2637–2637.
- Mittal V, Lo M, Damon LE, et al. Efficacy of venetoclax combination therapy with hypomethylating agents in patients with Relapsed acute myeloid leukemia after allogeneic hematopoietic cell transplantation. Blood. 2019;134(Suppl 1):5089–5089.
- Rausch CR, DiNardo CD, Maiti A, et al. Venetoclax Dosing in combination with Antifungal agents: Real World Experience in patients with acute myeloid leukemia. Blood. 2019;134(Suppl 1):2640–2640.
- Wei AH, Garcia JS, Borate U, et al. A phase 1b study evaluating the safety and efficacy of venetoclax in combination with azacitidine in treatment-Naïve patients with higher-risk myelodysplastic syndrome. Blood. 2019;134(Suppl 1):568–568.
- Winters A, Gutman JA, Purev E, et al. Venetoclax and azacitidine for older Newly diagnosed patients with acute myeloid leukemia: A Single-Institution Pilot study using Measurable Residual disease to Guide therapy. Blood. 2019;134(Suppl 1):2638–2638.
- Winters AC, Gutman JA, Purev E, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Advances. 2019;3(20):2911–2919.
- Zeidan AM, Pollyea DA, Garcia JS, et al. A phase 1b study evaluating the safety and efficacy of venetoclax As monotherapy or in combination with azacitidine for the treatment of Relapsed/refractory myelodysplastic syndrome. Blood. 2019;134(Suppl 1):565–565.
- Dinardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in Previously Untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Bewersdorf JP, Giri S, Wang R, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis. Haematologica. 2020. doi:10.3324/haematol.2019.242826
- Ji J, Chen M, Han B. Comparison of Hypomethylator monotherapy with Hypomethylator plus chemotherapy for intermediate/high-risk MDS or AML: A meta-analysis. J Cancer. 2020;11(10):2972–2980.
- Gao C, Wang J, Li Y, et al. Incidence and risk of hematologic toxicities with hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukopenia: A systematic review and meta-analysis. Medicine (Baltimore. 2018;97(34):e11860–e11860.