ABSTRACT
Objectives
To evaluate any association between pre-transfusion hemoglobin (Hb) levels and thalassemia complications and to identify the Hb cutoff values to predict thalassemia complications.
Methods
We performed a cross-sectional study in thalassemia patients who attended the Adult Hematology Clinic of the tertiary care University Hospital from October 2017 to October 2018. A point-biserial correlation was used to identify any association between Hb levels and thalassemia complications. A receiver operating characteristic (ROC) curve was used to identify the diagnostic ability of Hb levels to predict thalassemia complications and identify Hb cutoff values.
Results
Out of the 102 patients, there were 53 transfusion dependent thalassemia (TDT) patients and 49 non-transfusion dependent thalassemia (NTDT) patients. In theTDT group, Hb levels showed a negative correlation with severe hepatic iron overload and hypogonadism. The cutoff Hb levels to predict severe hepatic iron overload and hypogonadism were ≤7.01 and 6.81 g/dL, respectively, at which points the area under the ROC curve (AUC) were 0.721 and 0.708, respectively. In the NTDTgroup, Hb levels were negatively correlated with hepatic iron overload, osteoporosis, and pulmonary hypertension. The cutoff values of Hb levels to predict hepatic iron overload, osteoporosis, and pulmonary hypertension were ≤8.24, 7.16, and 7.16 g/dL, respectively, at which points the AUC were 0.923, 0.715, and 0.725, respectively.
Conclusions
Lower Hb level was associated with more frequent complications in both TDT and NTDT patients. The Hb cutoff levels to predict these complications were identified.
Introduction
Thalassemia is a common inherited Hb disorder in Thailand. Low pre-transfusion Hb level in thalassemia patients indicates ineffective erythropoiesis and hemolysis. This anemic state leads to chronic tissue hypoxia resulting in many complications. Adequate blood transfusion concomitant with effective iron chelation can prevent these complications and improve survival in thalassemia patients [Citation1,Citation2].
The Thalassemia International Federation (TIF) recommended that TDT patients should receive blood transfusions at a rate sufficient to maintain pre-transfusion Hb above 9–10.5 g/dL or higher (11–12 g/dL) for patients with cardiac complications [Citation1]. In NTDT patients, more frequent blood transfusions should be considered in some cases including growth failure, frequent hemolytic crisis or poor quality of life. Transfusion may also be considered for primary prevention in patients at a high risk of thrombosis, pulmonary hypertension, extramedullary hematopoiesis (EMH), or leg ulcers [Citation2]. In Northern Thailand where resources are limited, most TDT patients cannot access this hyper-transfusion regimen and many of NTDT patients who should be receiving frequent blood transfusions may not be doing so. Thus, the prevalence of thalassemia complications in this area may differ from more developed countries.
Pre-transfusion Hb was found to be associated with quality of life in both TDT and NTDT patients [Citation3]. Previous studies concerning thalassemia complications such as iron overload, EMH, thrombosis, pubertal development, growth rate and hypogonadism also found that pre-transfusion Hb level is one of the most frequently related parameters [Citation4–11]. However, the knowledge surrounding the association between pre-transfusion Hb and thalassemia complications is still limited.
This study had the primary endpoint of identifying the association between pre-transfusion Hb levels and thalassemia complications. The secondary endpoints were to identify the Hb cutoff values to predict thalassemia complications and to evaluate current prevalence of thalassemia complications.
Materials and methods
Population
We performed a cross-sectional study among thalassemia patients, aged 15 years or older, who attended the Adult Hematology Clinic of the tertiary care University Hospital from 1 October 2017–31 October 2018. All patients had been reviewed by a hematologist to confirm the diagnosis and type of thalassemia by Hb analysis using a high-performance liquid column chromatography method.
Data collection
Demographic characteristics including sex, age, co-morbidity, thalassemia type, transfusion status, iron chelation, and history of splenectomy were collected. Patients were classified as TDT or NTDT. TDT was defined as thalassemia disease requiring regular blood transfusion to survive while NTDT was defined as patients not requiring such lifelong regular transfusions for survival [Citation1,Citation2]. Previous laboratory tests including serum ferritin, magnetic resonance imaging (MRI) for cardiac T2* and MRI for liver iron concentration (LIC) were also collected. These data were obtained from our thalassemia database.
Pre-transfusion hemoglobin
Previous pre-transfusion Hb levels were collected for 10 consecutive times including at the time of enrollment. The Hb levels which decreased more than 2 g/dL from baseline due to infection or blood loss were excluded. We collected pre-transfusion Hb levels every 3 months. For NTDT patients scheduled for follow up less frequently than every 3 months, we collected previous pre-transfusion Hb levels every visit until 10 values were recorded.
Thalassemia complications
Thalassemia complications at the time of enrollment were reviewed from our thalassemia database. The complications included in this study were iron overload characterized by serum ferritin level, hepatic iron overload, cardiac iron overload, hypothyroidism, hypogonadism, adrenal insufficiency, diabetes mellitus, cholelithiasis, cirrhosis, hepatocellular carcinoma (HCC), pulmonary hypertension, cardiomyopathy, thrombosis, osteoporosis, EMH, and leg ulceration. These complications were defined as shown in supplementary Table 1.
All thalassemia patients in this cohort had been routinely evaluated by laboratory investigation to screen for thalassemia complications. A liver function test had been carried out every 3 months. Endocrine function was screened by fasting blood sugar and thyroid function test which were done annually. Plain x-ray or MRI had been done for suspected cases of EMH. Echocardiography had been carried out in patients who were suspect pulmonary hypertension or cardiomyopathy. Vascular investigation had been carried out for suspected cases of thrombosis from any site. Abdominal radiology was done for suspected cases of cholelithiasis, cirrhosis or HCC. Bone densitometry had been carried out in suspected cases of osteoporosis. Serum cortisol was done for the cases with suspected adrenal insufficiency. MRI for cardiac T2* and LIC had been done at least once in all patients.
Statistical analysis
The association between Hb levels and thalassemia complications were analyzed by point-biserial correlation. The complications which were found to be associated with Hb levels were entered in the ROC curve analysis to identify the diagnostic efficacy of Hb levels to predict thalassemia complications and identify Hb cutoff values. The prevalence of thalassemia complications and other descriptive results of categorical variables are reported as numbers and percentages. Previous pre-transfusion Hb levels and other continuous variables are reported as mean and standard deviations (SD). The student’s t-test was used to compare continuous variables. P-values less than 0.05 were considered as statistically significant. Univariate analysis was use to evaluate factors associated with thalassemia complications. All factors which p-value < 0.25 were entered into multivariate analysis. All data analysis was performed using the SPSS program, version 26.0.
Results
Patients
A total of 102 patients were included in this study. The main demographic and clinical characteristics of the patients are provided in . There were 36 (35.3%) male patients. The mean age was 32.11 ± 8.98 years. Eighty-two (80.4%) patients had beta-thalassemia disease. Fifty-three (52.0%) patients were TDT. Fifty-eight (56.9%) patients had had splenectomies.
Table 1. Demographic and clinical characteristics of the study patients.
Prevalence of thalassemia complications
shows the prevalence of thalassemia complications in this study. Complications occurred in 92 (90.2%) patients. Apart from iron overload characterized by serum ferritin and hepatic iron overload, the three most common complications in both TDT and NTDT patients were osteoporosis, cholelithiasis, and EMH. The complications that occurred significantly more in TDT than in NTDT patients were iron overload characterized by serum ferritin, hepatic iron overload, hypothyroidism, and hypogonadism. The complication that developed significantly more frequently in NTDT patients was pulmonary hypertension.
Table 2. Prevalence of thalassemia complications.
Clinical factors affecting thalassemia complications
The results of the univariate and multivariate logistic regression analysis for risk factors of thalassemia complications are shown in supplementary tables 4 and 5. From the multivariate analysis, increasing age (odds ratio [OR] 95% confidence interval [CI]:1.078, [1.001–1.160]) and low Hb value (OR 95% CI: 0.358, [0.148–0.864]) was significantly associated with severe hepatic iron overload in TDT patients. Low Hb value (OR 95% CI: 0.380, [0.145–0.994]) was significantly associated with hypogonadism in TDT patients. In NTDT patients, Low Hb (OR 95% CI: 0.120, [0.21–0.701]) value was significantly associated with hepatic iron overload. High LIC value (OR 95% CI: 1.589, [1.108–2.280]) and low Hb value (OR 95% CI: 0.146, [0.31–0.688]) were significantly associated with osteoporosis in NTDT patients. Male sex (OR 95% CI: 0.039, [0.002–0.641]) and low Hb value (OR 95% CI: 0.157, [0.031–0.809]) were significantly associated with pulmonary hypertension in NTDT patients.
Association between pre-transfusion Hb and thalassemia complications
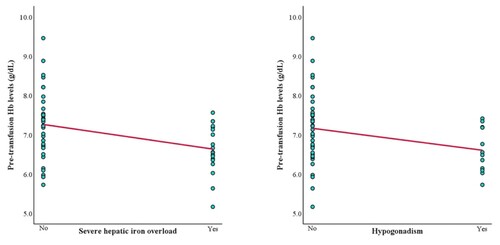
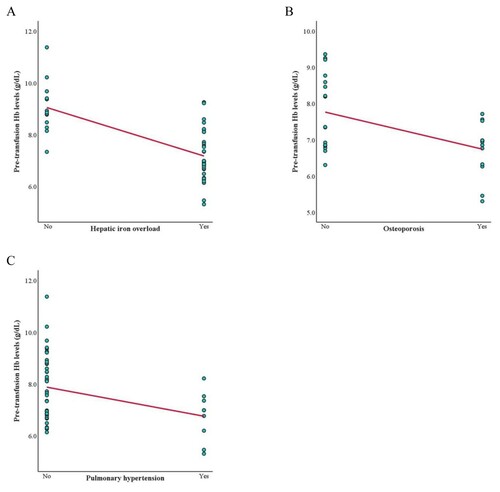
To clarify the difference between TDT and NTDT complications, we analyzed the association between pre-transfusion Hb and thalassemia complications in each group separately. In the TDT group, Hb levels had a negative correlation with severe hepatic iron overload (LIC ≥15 mg Fe/g dry weight) and hypogonadism (r = −0.363 and −0.282; Supplementary Table 2). In the NTDT group, Hb levels had a negative correlation with hepatic iron overload, osteoporosis, and pulmonary hypertension (r = −0.667, −0.494, and −0.350; Supplementary Table 3). The scatter plots illustrating Hb levels and these complications are shown in and .
Hemoglobin cutoff values
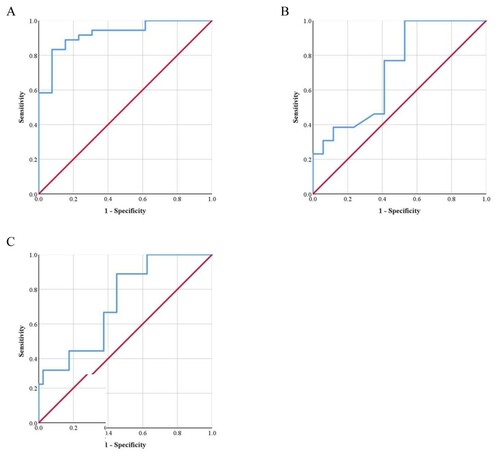
In the TDT group, an Hb level ≤7.01 g/dL was the cutoff to predict severe hepatic iron overload (AUC = 0.721, 68.4% sensitivity, 61.8% specificity, (A)). An Hb level ≤6.81 g/dL was the cutoff to predict hypogonadism (AUC = 0.708, 66.7% sensitivity, 63.4% specificity, (B)).
Figure 3. ROC curve for predicting (A) severe hepatic iron overload (B) hypogonadism in TDT patients based on pre-transfusion Hb levels.
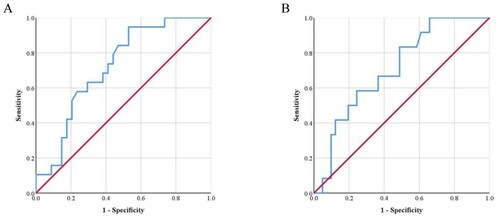
In the NTDT group, an Hb level ≤8.24 g/dL was the cutoff to predict hepatic iron overload (AUC = 0.923, 88.9% sensitivity, 84.6% specificity, (A)). An Hb level ≤7.16 g/dL was the cutoff to predict osteoporosis (AUC = 0.715, 76.9% sensitivity, 58.8% specificity, (B)). Hb level ≤7.16 g/dL was the cutoff to predict pulmonary hypertension (AUC = 0.725, 66.7% sensitivity, 62.5% specificity, (C)).
Discussion
Despite the therapeutic use of blood transfusion and iron chelation for several years in Thailand, our study shows that the prevalence of iron overload and other thalassemia related complications are still high. The results from this cohort show that the prevalence of iron overload by serum ferritin level was 88.7% in TDT patients and 47.9% in NTDT patients. The prevalence of hepatic iron overload indicated by LIC measured by MRI in this cohort was 88.7% in TDT and 73.5% in NTDT patients compared with 83.6% [Citation12] and 63.6% [Citation13] respectively in previous studies. The three most common complications in both TDT and NTDT patients apart from iron overload characterized by serum ferritin and hepatic iron overload were osteoporosis, cholelithiasis, and EMH.
The complications that were found to occur significantly in the TDT group were iron overload characterized by serum ferritin, hepatic iron overload, hypothyroidism, and hypogonadism. Whereas, the only complication found to be significant in the NTDT group was pulmonary hypertension. These results were consistent with those from previous studies [Citation14,Citation15]. However, the results which differed from previous studies [Citation14–16] showed that osteoporosis, thrombosis, and EMH were more frequently found in the NTDT group. As our patients were not routinely screened for BMD, the missing data is quite high in the NTDT group, therefore, the prevalence of osteoporosis in NTDT patients may be under representative. Probably due to the low prevalence of thrombosis in Thai patients generally [Citation17], the prevalence of thrombosis in this study was low in both the TDT and NTDT group and there was no significant difference in prevalence between both groups. The reason for the high prevalence of EMH in TDT patients in this study might be due to an inadequate rate of transfusion. This is demonstrated by the lower mean pre-transfusion Hb value (7.03 ± 0.84 g/dL) compared with the level recommended by TIF (above 9–10 g/dL) [Citation1].
In the TDT group there was a lower prevalence of cardiac iron overload than that reported by the CORDELLIA study [Citation12] in 2012 and that carried out by Tantiworawit et al [Citation18] in 2012 (7.5%, 36.6%, and 11.0% respectively). This result might be due to greater effectiveness of iron chelation therapy than that nearly ten years ago. The prevalence of cardiomyopathy was comparable with studies in Dubai [Citation19] and North East Thailand [Citation20]. Diabetes mellitus and hypothyroidism were more prevalent than in previous studies [Citation14,Citation15,Citation19–22].
In the NTDT group, our study was comparative to the OPTIMAL CARE study [Citation8] in that there was a higher prevalence of cholelithiasis and pulmonary hypertension but a lower prevalence of leg ulceration. The prevalence of cirrhosis and HCC were comparable with reports from the study by Mancuso et al. [Citation23]. In the three patients who had cirrhosis, one patient had a chronic viral hepatitis B infection and one had a chronic viral hepatitis C infection. One patient who developed HCC also had a chronic hepatitis C infection and cirrhosis concomitantly.
We demonstrated a negative correlation between pre-transfusion Hb and some thalassemia complications. In the TDT group, an association was found with hypogonadism and the level of LIC ≥15 mg Fe/g dry weight. This cutoff LIC level indicates a severe hepatic iron overload causing severe adverse effects, including liver fibrosis and dysfunction [Citation24]. In the NTDT group, an association was found with hepatic iron overload, osteoporosis, and pulmonary hypertension. These results were consistent with findings from previous studies. A study by Tantiworawit, et al [Citation9] found that an Hb level < 7 g/dL was a significant risk factor of iron overload in NTDT patients. The OPTIMAL CARE study also noted that the NTDT patients who received more frequent transfusion therapy had a level of protection against development of pulmonary hypertension [Citation8]. A study in TDT patients [Citation25] also demonstrated that a low Hb level was one of the risk factors for the development of hypogonadism. These results were supported by multiple theories associated with the anemic state including erythroid marrow expansion, hypercoagulable state, and iron overload by increasing gastrointestinal (GI) tract absorption. Interestingly, iron overload in TDT patients which occurs as a direct result of blood transfusion tended to be found in patients who had lower pre-transfusion Hb levels. Moreover, the correlation was statistically significant in cases of severe hepatic iron overload. So, we presumed that TDT patients who did not receive adequate blood transfusion can still develop iron overload through other mechanisms besides directly from transfusion, for example increasing GI tract absorption.
The risk factors associated with complications in thalassemia are multi-factorial. In addition to the lower Hb level other factors also contribute to complications. Iron overload caused by blood transfusion is one of the most important risk factors as is increased gastrointestinal tract absorption reflected by low Hb level. High ferritin level and LIC showed significant correlations with complications and survival in TDT patients [Citation26,Citation27]. In NTDT, iron overload as reflected by ferritin level and LIC shows a correlation with complications [Citation1,Citation8,Citation11,Citation18,Citation28]
This study also identifies the Hb cutoff values predicting complications associated with thalassemia. TDT patients with an Hb level of less than 6.81 and 7.01 g/dL tended to develop hypogonadism and severe hepatic iron overload. NTDT patients with Hb levels of less than 8.24, 7.16, and 7.16 g/dL tended to develop hepatic iron overload, osteoporosis, and pulmonary hypertension respectively. All of the AUC values were more than 0.5. These results can be effectively integrated into current practice in our region despite limited resources. If it is not possible to maintain pre-transfusion Hb level above 9–10 g/dL in TDT patients in line with TIF guidelines [Citation1], at least we should maintain it above 7.01 g/dL to reduce the risk of hypogonadism and severe hepatic iron overload. Even though we do not use Hb level to inform the need for transfusion in cases of NTDT, our Hb cutoff values may be used as indicators for complications. Thus, increasing awareness of maintaining the pre-transfusion Hb level above 8.24 g/dL could be beneficial.
Our study had some limitations. First, this is the cross-sectional study which could not address the causal effect. Second, data concerning thalassemia complications in this study were obtained by retrospective review and some complications were not routinely screened for and some were not diagnosed by gold standard methods. Therefore, the prevalence of some complications may be under represented. Finally, using the mean value of ferritin, LIC and cardiac T2* to represent the current iron overload and cardiac iron overload status could be modified by the blood transfusion and iron chelation therapy during that period.
In conclusion, lower pre-transfusion Hb level was associated with more frequent complications in both TDT and NTDT patients. Hb cutoff levels to predict these complications were identified. Optimal blood transfusion and effective iron chelation should be used to prevent these complications.
Supplemental Material
Download MS Word (40.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cappellini M-D, Cohen A, Porter J, et al. Guidelines for the management of transfusion dependent thalassaemia (TDT): Thalassaemia International Federation Nicosia, Cyprus; 2014.
- Taher A, Vichinsky E, Musallam K, et al. Guidelines for the management of non transfusion dependent thalassaemia (NTDT): Thalassaemia International Federation, Nicosia, Cyprus; 2013.
- Thavorncharoensap M, Torcharus K, Nuchprayoon I, et al. Factors affecting health-related quality of life in Thai children with thalassemia. BMC Hematol. 2010;10(1):1.
- Cazzola M, Borgna-Pignatti C, Locatelli F, et al. A moderate transfusion regimen may reduce iron loading in beta-thalassemia major without producing excessive expansion of erythropoiesis. Transfusion. 1997;37(2):135–140.
- Cazzola M, Stefano PD, Ponchio L, et al. Relationship between transfusion regimen and suppression of erythropoiesis in β-thalassaemia major. Br J Haematol. 1995;89(3):473–478.
- Malik S, Syed S, Ahmed N. Complications in transfusion–dependent patients of ß-thalassemia major: a review. Pak J Med Sci. 2009;25(4):678–682.
- Saxena A. Growth retardation in thalassemia major patients. Int J Hum Genet. 2003;3(4):237–246.
- Taher AT, Musallam KM, Karimi M, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886–1892.
- Tantiworawit A, Charoenkwan P, Hantrakool S, et al. Iron overload in non-transfusion-dependent thalassemia: association with genotype and clinical risk factors. Int J Hematol. 2016;103(6):643–648.
- Ragab LA, Hamdy MM, Shaheen IA, et al. Blood transfusion among thalassemia patients: a single Egyptian center experience. Asian J Transfus Sci. 2013;7(1):33.
- Winichakoon P, Tantiworawit A, Rattanathammethee T, et al. Prevalence and risk factors for complications in patients with Nontransfusion dependent alpha- and beta-thalassemia. Anemia. 2015;2015:793025.
- Aydinok Y, Porter JB, Piga A, et al. Prevalence and distribution of iron overload in patients with transfusion-dependent anemias differs across geographic regions: results from the CORDELIA study. Eur J Haematol. 2015;95(3):244–253.
- Taher AT, Porter JB, Viprakasit V, et al. Deferasirox reduces iron overload significantly in nontransfusion-dependent thalassemia: 1-year results from a prospective, randomized, double-blind, placebo-controlled study. Blood. 2012;120(5):970–977.
- Inati A, Zeineh N, Isma'Eel H, et al. Β-Thalassemia: the Lebanese experience. Clin Lab Haematol. 2006;28(4):217–227.
- Taher A, Isma'eel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006;37(1):12–20.
- Hashemieh M, Azarkeivan A, Radfar M, et al. Prevalence of osteoporosis among thalassemia patients from Zafar adult thalassemia clinic, Iran. Iran J Blood Cancer. 2014;6(3):143–148.
- Angchaisuksiri P, Atichartakarn V, Aryurachai K, et al. Risk factors of venous thromboembolism in Thai patients. Int J Hematol. 2007;86(5):397–402.
- Tantiworawit A, Tapanya S, Phrommintikul A, et al. Prevalence and risk factors for cardiac iron overload and cardiovascular complications among patients with thalassemia in Northern Thailand. Southeast Asian J Trop Med. 2016;47(6):1335–1342.
- Belhoul KM, Bakir ML, Kadhim AM, et al. Prevalence of iron overload complications among patients with β-thalassemia major treated at Dubai thalassemia Centre. Ann Saudi Med. 2013;33(1):18–21.
- Teawtrakul N, Jetsrisuparb A, Pongudom S, et al. Epidemiologic study of major complications in adolescent and adult patients with thalassemia in Northeastern Thailand: the E-SAAN study phase I. Hematology. 2018;23(1):55–60.
- Li CK, Luk CW, Ling SC, et al. Morbidity and mortality patterns of thalassaemia major patients in Hong Kong: retrospective study. Hong Kong Med J. 2002;8(4):255–260.
- Cunningham MJ, Macklin EA, Neufeld EJ, et al. Complications of β-thalassemia major in North America. Blood. 2004;104(1):34–39.
- Mancuso A, Sciarrino E, Concetta Renda M, et al. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. 2006;30(1):119–124.
- Poggiali E, Cassinerio E, Zanaboni L, et al. An update on iron chelation therapy. Blood Transfusion. 2012;10(4):411.
- Albu A, Barbu CG, Antonie L, et al. Risk factors associated with hypogonadism in β–thalassemia major patients: predictors for a frequent complication of a rare disease. Postgrad Med. 2014;126(5):121–127.
- Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous β-thalassemia. N Engl J Med. 1994;331(9):574–578.
- Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–1193.
- Inthawong K, Charoenkwan P, Silvilairat S, et al. Pulmonary hypertension in non-transfusion-dependent thalassemia: correlation with clinical parameters, liver iron concentration, and non-transferrin-bound iron. Hematology. 2015;20(10):610–617.