ABSTRACT
Objective
To identify potential mutations of the FXII gene (F12) in a consanguineous marriage family with hereditary coagulation factor XII (FXII) deficiency, and it will improve the understanding of the pathogenesis involved in the disease.
Clinical presentation
The proband was a 58-year-old male who had chronic gastritis. He was found to have a significantly prolonged activated partial thromboplastin time (APTT) at 101.0s (reference range, 29.0–43.0 s) before stomachendoscopy.
Techniques
The coagulation factor XII activity (FXII:C) and FXII antigen (FXII:Ag) were measured by one-stage clotting assay and enzyme-linked immunosorbent assay, respectively. The F12 gene was amplified by polymerase chain reaction and sequenced. Mutation sites were further confirmed by reverse sequencing. The conservatism and possible impact of the amino acid substitution were analyzed by multiple bioinformatics tools, as well as 3D protein model analysis.
Results
The proband had a prolonged APTT (101.0 s), whose FXII:C and FXII:Ag were obviously reduced, both at 1.0% (normal range, 72–113%). Gene sequencing revealed that he carried a homozygous missense mutation of Met527Ile. Family study showed that his mother, son and daughter carried a heterozygous Met527Ile. Bioinformatics and model analysis of the mutation indicated that Met527Ile may be detrimental and potentially alters the structure and the function of the protein.
Conclusion
The novel mutation Met527Ile could potentially account for the reduced activity of FXII in this family.
Introduction
Human blood coagulation factor XII (FXII) (Hageman factor) is a serine protease precursor that primarily synthesized by hepatocytes and secreted into plasma with a concentration of approximately 40 μg/mL [Citation1]. FXII is a single-chain glycoprotein composed of 596 amino acid residues. Following contact with negatively charged surfaces, FXII is cleaved into FXIIa, which consists of an N-terminal heavy chain and a C-terminal light chain linked by a disulfide bond [Citation2]. FXIIa initiates the rapid intrinsic coagulation pathway via activation of factor XI. Additionally, FXIIa plays an essential role in the fibrinolysis pathway, complement activation and inflammatory response [Citation3,Citation4]. Furthermore, recent studies have obtained more insight into the influence of FXII on cell biology. Zymogen FXII also has biologic activity related to cell protection and repair, such as endothelial cell, fibroblast, neutrophil and dendritic cell [Citation5].
The gene encoding FXII, F12, is located on the chromosomal band 5q33-qter and contains 14 exons with a size of 12 kb [Citation6]. Congenital FXII deficiency is a rare, autosomal recessive disorder, mainly caused by various mutations in the F12, with an incidence of approximately 1/1,000,000 [Citation7]. According to the FXII antigen (FXII:Ag) level, FXII deficiency can be classified into two categories: a cross-reacting material (CRM)-positive group (normal FXII:Ag levels) and a CRM-negative group (low FXII:Ag levels), of which the latter is more common [Citation8]. Humans with congenital FXII deficiency are clinically asymptomatic, but mostly screened by chance due to prolonged activated partial thromboplastin time (APTT) prior to surgery. FXII-deficient individuals have normal hemostatic capacity and rarely suffer from bleeding tendency [Citation9]. In addition, several animal studies indicated that FXII-deficient mice were protected against thrombosis [Citation10,Citation11]. Therefore, targeting FXIIa offers a promising perspective for developing safe anticoagulants devoid of bleeding risks.
In this paper, we described a patient with FXII deficiency from a consanguineous marriage family. The phenotype and genotype of the proband and his families were studied. A novel missense mutation (c.1638G > A(Met527IIe)) in exon 13 of F12 gene was identified.
Patients and methods
Patients
The proband, a 58-year-old Chinese male, was admitted to hospital for chronic gastritis. He had no bleeding or thromboembolic events previously and had no documented history of liver disease. He was not taking any oral anticoagulants. Routine coagulation screening before stomach endoscopy showed markedly prolonged APTT at 101.0 s (reference range 29.0–43.0 s). The prolonged APTT prompted a further investigation of the intrinsic factor assays, Which revealed significantly reduced FXII activity (FXII:C) as well as FXII:Ag, both at 1.0% (normal range, 72–113%). Other coagulation indices were within normal limits. FXII inhibitor and lupus anticoagulant (LA) were also negative. Therefore, the proband was diagnosed with FXII deficiency. Since deficiency of FXII does not cause bleeding disorder [Citation9], the endoscopy was well performed on schedule without complications.
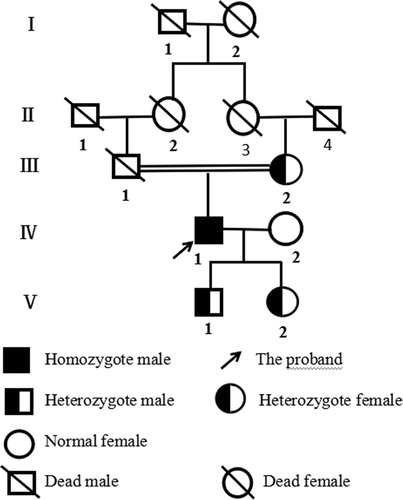
An investigation was made on the family history. Consanguineous marriage was confirmed in his parents, as his paternal grandmother and maternal grandmother were sister-German (). A total of five members of three generations were recruited in this study. His mother, son and daughter showed slightly extended APTT, and intermediate level of FXII:C and FXII:Ag. His wife was found without any abnormal result. Ethical approval was obtained from the Ethics Committee of the First Hospital Affiliated to Wenzhou Medical University (China).
Methods
Coagulation tests
Prothrombin time (PT), APTT, fibrinogen (FIB), FXII:C, factor VIII activity (FVIII:C), factor IX activity (FIX:C), factor XI activity (FXI:C) and LA were determined by a clotting assay on STAGO STA-R automated analyzer with matched commercially available kits. FXII:Ag was performed with enzyme-linked immunosorbent assay kit (Changfeng, Wenzhou, China). The reference ranges of these indices in our laboratory were established with 100 healthy subjects.
DNA analysis
Genomic DNA was extracted using DNA blood-extracting kits (Tiangen, Beijing, China). Seven sets of primers which covered the entire coding region of the F12 gene have been described previously [Citation12]. Polymerase chain reaction was processed on the Applied Biosystem Thermal Cycler 2720 (ABI, Foster City, USA). The amplified products were purified and sequenced at Sunsoon BIO-Technology Corporation (Shanghai, China). Potential mutation site was confirmed by reverse sequencing. Hundred individuals were selected to exclude the polymorphism.
Bioinformatics analysis
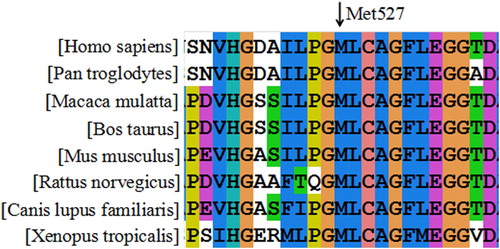
The conservation of the residue Met527 among homo sapiens and seven homologous species (Pan troglodytes, Macaca mulatta, Bos taurus, Mus musculus, Rattus norvegicus, Canis lupus familiaris, Xenopus tropicalis) was checked by multiple sequence alignment software ClustalX-2.1-win (HomoloGene, http://www.ncbi.nlm.nih.gov/homologene). The possible impact of the amino acid substitution Met527Ile was assessed with four online bioinformatics tools (Mutation Taster, PolyPhen-2, PROVEAN and SIFT).
Protein model analysis
Structural analysis of the mutant protein was carried out with Swiss-Pdb Viewer version 4.0.1. (Swiss Institute of Bioinformatics, Lausanne, Switzerland) and PIC programs (Protein Interactions Calculator, http://pic.mbu.iisc.ernet.in) based on the three-dimensional structure in the Protein Data Bank (PDB, http://www.rcsb.org/pdb/home/home.do, PDB ID: 4XE4).
Results
Coagulation tests
The phenotypic characteristics of the family members were summarized in . The proband had severely prolonged APTT, extremely reduced FXII:C and FXII:Ag, which were 101.0 s, 1% and 1.0%, respectively. His mother, son and daughter showed slightly extended APTT, around the upper limit of reference range, as well as intermediate level of FXII:C and FXII:Ag. Levels of FVIII:C, FIX:C, FXI:C and LA of the family members were all within normal limits (data not shown).
Table 1. Phenotypes and genotypes of the family members.
DNA analysis
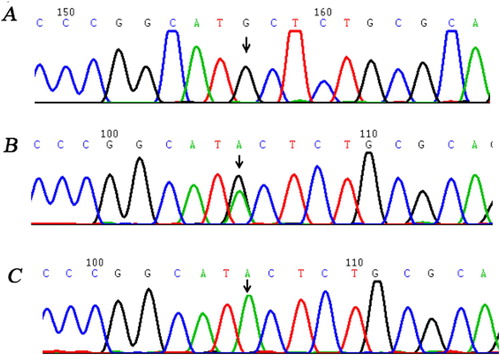
Genetic studies revealed that the proband carried a homozygous missense mutation c.1638G > A in exon 13 leading to a substitution of Methionine 527 by Isoleucine (Met527Ile). His mother, son and daughter were heterozygotes for Met527Ile. Besides, since 100 healthy controls were all wild type, the Met527Ile missense mutation was not considered as a polymorphism ().
Bioinformatics analysis
The results of ClustalX software declared that Met527 was highly conserved among the homologous species (). The predicting outcomes of Met527Ile with ‘PolyPhen-2’, ‘PROVEAN’, ‘SIFT’ and ‘Mutation Taster’ were consistent, which were ‘Probable damaging’, ‘Deleterious’, ‘Damaging’ and ‘Disease causing’ with scores of ‘0.999’, ‘−3.74’, ‘0.000’ and ‘0.999’, respectively.
Protein model analysis
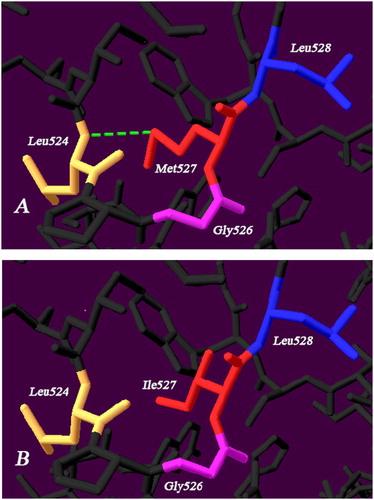
Model analyzing demonstrated that the Met527 had a hydrogen bond with Leu524 in the wild type. Once substituted by Ile527, the previous hydrogen bond disappeared ().
Discussion
Congenital FXII deficiency is an uncommon monogenic disease, characteristic of evidently prolonged APTT and decreased FXII levels. The proband who was admitted to the hospital due to chronic gastritis was incidentally found to have greatly extended APTT at 101.0 s, which can be corrected with normal mixing plasma. Following assays showed that the FXII:C and FXII:Ag levels decreased equivalently both at 1.0%, and other causes were excluded, suggesting a diagnosis of CRM-negative FXII deficiency. DNA analysis revealed that the proband carried a homozygous missense mutation of Met527Ile. Family study showed that his mother, son and daughter carried a heterozygous Met527Ile. Just as the autosomal recessive inheritance pattern, the mutant gene was inherited from his parents, and then passed on to his son and daughter. Therefore, we considered that the missense mutation of Met527Ile could potentially account for the reduction of FXII:C and FXII:Ag in this family. Notably, this is the first reported case of Met527Ile mutation in the world.
On the basis of the previous observation, homozygous or compound heterozygous carriers always be associated with almost no FXII:C, while heterozygotes were moderately deficient in serum FXII:C [Citation13]. Our study was consistent with these finding. All the genetic results of the family members were comparable with the levels of FXII:C and FXII:Ag. It is interesting that none of the mutant members had a history of bleeding or thrombotic episodes. Subjects with a deficiency in FXII were asymptomatic and do not undergo spontaneous or injury-related bleeding, which was quite distinct from the other coagulation factor deficiency [Citation14,Citation15]. The relation between FXII deficiency and thrombotic events was controversial. Early findings indicated that FXII deficiency may increase the risk of thrombosis [Citation16]. However, subsequent studies came up with reverse results [Citation10,Citation11]. More recent investigations showed FXII deficiency was not associated with an increased risk of arterial or venous thrombosis [Citation17,Citation18]. The role of FXII in thrombosis and hemostasis still remains poorly defined.
Basing on the result of conservation analysis (), Met527 residue was highly conserved among the homologous species, indicating Met527 plays a critical part in the protein. Such conclusion was further supported by the forecasting outcomes of these four online bioinformatics software, which accordantly described Met527Ile mutation as a deleterious mutation and could impair the protein function.
The novel mutation Met527IIe giving rise to a CRM-negative FXII deficiency, located in the catalytic domain of the light chain. As a member of serine proteases, the structure of catalytic triad His393-Asp442-Ser544 is crucial for the FXII enzyme activity. However, mutations in this region could cause both CRM-negative and -positive phenotypes [Citation12,Citation13]. To our knowledge, the intracellular degradation mechanism of CRM-negative FXII mutation involves the targeted degradation of protein quality-control system in endoplasmic reticulum (ER) and proteasome-mediated degradation of the mutant proteins in pre-Golgi compartment [Citation19,Citation20]. Moreover, the mutation Glu521Lys had been reported to be normally transcribed and synthesized at ER, but an extensive degradation of mutant occurred in the pre-Golgi compartment, thus, contributed to markedly decreased FXII activity and antigen levels [Citation8]. Since Glu521 was close to the mutation Met527Ile in our case, we speculated that it is also the case with the misfolded protein induced by Met527Ile, which has the same secretion impairment as Glu521Lys. Meanwhile, the analysis of the molecular structure demonstrated that replacement of Met527 with Ile527 resulted in disappearance of a hydrogen bond between Leu524-Met527, which may bring about harmful interactions with surrounding amino acids and damage the natural space conformation of the protein. The substitution may impair intracellular protein processing and eventually lead to a CRM-negative FXII deficiency. Zhang et al had previously shown Met527Thr mutation to be the cause of FXII deficiency in a family, which is the same site but different mutant amino acid from our study [Citation21].
In summary, we identified a novel mutation Met527Ile in the congenital FXII-deficient family and expounded the possible pathogenic mechanism. In light of these findings, we concluded that the mutation Met527Ile is the molecular basis of the CRM-negative FXII deficiency. The precise mechanism remains to be further studied.
Acknowledgments
The authors thank the patients and their family members for their consent to take part in our study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Weidmann H, Heikaus L, Long AT, et al. The plasma contact system, a protease cascade at the nexus of inflammation, coagulation and immunity. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 Pt B):2118–2127.
- Renné T, Schmaier AH, Nickel KF, et al. In vivo roles of factor XII. Blood. 2012;120(22):4296–4303.
- Schmaier AH. The elusive physiologic role of factor XII. J Clin Invest. 2008;118(9):3006–3009.
- Müller F, Renné T. Novel roles for factor XII-driven plasma contact activation system. Curr Opin Hematol. 2008;15(5):516–521.
- Schmaier AH, Stavrou EX. Factor XII – what's important but not commonly thought about. Res Pract Thromb Haemost. 2019;3(4):599–606.
- Cool DE, MacGillivray RT. Characterization of the human blood coagulation factor XII gene. Intron/exon gene organization and analysis of the 5'-flanking region. J Biol Chem. 1987;262(28):13662–13673.
- Fernandes HD, Newton S, Rodrigues JM. Factor XII Deficiency Mimicking Bleeding Diathesis: a unique presentation and diagnostic pitfall. Cureus. 2018;10(6):e2817.
- Jin P, Jiang W, Yan H, et al. Novel mutations in congenital factor XII deficiency. Front Biosci (Landmark Ed). 2016;21:419–429.
- Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5(12):2323–2329.
- Worm M, Kohler E C, Panda R, et al. The factor XII a blocking antibody 3F7: a safe anticoagulant with anti-inflammatory activities. Ann Transl Med. 2015;3(17):247.
- Travers RJ, Shenoi RA, Kalathottukaren MT, et al. Nontoxic polyphosphate inhibitors reduce thrombosis while sparing hemostasis. Blood. 2014;124(22):3183–3190.
- Cheng X, Yang L, Huang G, et al. Genetic analysis of a hereditary factor XII deficiency pedigree of a consanguineous marriage due to a homozygous F12 gene mutation: Gly341Arg. Hematology. 2017;22(5):310–315.
- Schloesser M, Zeerleder S, Lutze G, et al. Mutations in the human factor XII gene. Blood. 1997;90(10):3967–3977.
- Hao X, Cheng X, Wang Y, et al. A novel gene insertion combined with a missense mutation causing factor VII deficiency in two unrelated Chinese families. Blood Coagul Fibrinolysis. 2015;26(6):687–690.
- Wang Y, Zhu L, Ye L, et al. Factor V deficiency caused by a novel nonsense mutation (Gln2031stop) in a Chinese patient. Blood Coagul Fibrinolysis. 2014;25(3):283–285.
- Zeerleder S, Schloesser M, Redondo M, et al. Reevaluation of the incidence of thromboembolic complications in congenital factor XII deficiency–a study on 73 subjects from 14 Swiss families. Thromb Haemost. 1999;82(4):1240–1246.
- Girolami A, Ferrari S, Cosi E, et al. Heterozygous FXII deficiency is not associated with an increased incidence of thrombotic events: results of a long term study. Blood Cells Mol Dis. 2019;77:8–11.
- Girolami A, Ferrari S, Cosi E, et al. Thrombotic events in severe FXII deficiency in comparison with unaffected family members during a long observation period. J Thromb Thrombolysis. 2019;47(3):481–485.
- Kondo S, Tokunaga F, Kawano S, et al. Factor XII Tenri, a novel cross-reacting material negative factor XII deficiency, occurs through a proteasome-mediated degradation. Blood. 1999;93(12):4300–4308.
- Ishii K, Oguchi S, Moriki T, et al. Genetic analyses and expression studies identified a novel mutation (W486C) as a molecular basis of congenital coagulation factor XII deficiency. Blood Coagul Fibrinolysis. 2004;15(5):367–373.
- Zhang H, Liu S, Lin C, et al. Compound heterozygous mutations Glu502Lys and Met527Thr of the FXII gene in a patient with factor XII deficiency. Hematology. 2019;24(1):420–425.