ABSTRACT
Objectives
Ras-related dexamethasone-induced 1 (RASD1) is abnormally expressed in many solid cancers. However, its potential role in adults with B-cell acute lymphoblastic leukemia (B-ALL) is unclear. Therefore, we aim to clarify the abnormal expression of the tumor-associated biomarker, RASD1, as a potential target for diagnosis and prognosis in adult Philadelphia-negative B-ALL.
Methods
The expression of RASD1 was detected with RT-qPCR in 92 adults with de novo Ph-negative B-ALL and 40 healthy controls. The correlation between RASD1 transcript levels and relapse was assessed.
Results
RASD1 transcript levels in patients with Ph-negative B-ALL (median 81.76%, range 0.22%-1824.52%) were significantly higher than those in healthy controls (7.59%, 0.46%-38.66%; P<0.0001). Patients with low RASD1 transcript levels had a lower 5-year relapse-free survival (RFS, 47.5% [32.9%, 62.1%] vs. 63.1% [49.0%, 77.2%]; P = 0.012) and a higher 5-year cumulative incidence of relapse (CIR, 52.0% [37.4%, 66.6%] vs. 36.2% [22.2%, 50.2%]; P = 0.013) especially in patients receiving chemotherapy only. Multivariate analysis showed that a low RASD1 transcript level was an independent risk factor for RFS (HR = 2.938 [1.427, 6.047], P = 0.003) and CIR (HR = 3.367 [1.668, 6.796], P = 0.001) in patients with Ph-negative B-ALL.
Conclusions
RASD1 transcript levels were significantly higher in patients with Ph-negative B-ALL and a low RASD1 transcript level was independently correlated with increased relapse risk.
1. Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is a hematologic malignancy characterized by a blockade of B-cell differentiation and excessive accumulation of lymphoblasts and progenitors [Citation1]. With advances in risk assessment and treatment regimens, the cure rate for adults with B-ALL has improved with complete remission (CR) rate greater than 80%. However, half of patients experience relapse and survival outcomes remain unsatisfactory [Citation2]. Various factors have been identified in risk stratification, but many relapses occur in patients who initially present no known adverse risk features, suggesting the heterogeneous biological and clinical characteristics of adult B-ALL [Citation3]. Therefore identifying novel prognostic biomarkers may be particularly helpful for patients yet unclassified by the known risk factors and may facilitate further refinement of risk stratification and risk-adapted therapy [Citation4].
Ras-related dexamethasone-induced 1 (RASD1) is located on chromosome 17p11.2 and encodes a member of the Ras superfamily of small GTPases [Citation5]. RASD1 is expressed in a variety of tissues and is involved in diverse physiological activities, such as cell proliferation, adipogenesis, and neuronal signal transduction [Citation6,Citation7]. Its abnormal expression has been found in various tumors, such as osteosarcoma, prostate cancer, renal cell carcinoma and glioma and high RASD1 expression was found to be correlated with better survival in astrocytoma patients [Citation8–12]. In addition, RASD1 was reported to play an important regulatory role in tumor growth and expansion. It was found to inhibit cell growth and induce apoptosis in breast cancer, lung cancer and prostate cancer cells and to inhibit cell migration and invasion in glioma cells [Citation10,Citation11,Citation13,Citation14]. In addition, RASD1 was also found to play a regulatory or inhibitory role in the activity of B cells [Citation15].
Our microarray analysis (data unpublished) showed that RASD1 is expressed at higher levels in B-ALL patients than in healthy controls. We next searched the freely available immune cell gene expression database (http://immusort.bjmu.edu.cn/Account/ImmuSort.html) and found that RASD1 transcript levels were upregulated in B-ALL cells compared with normal B-cells. A recent study showed that high RASD1 transcript levels predicted poor survival in 53 patients with B-ALL [Citation16]. However, the prognostic value of RASD1 transcript levels should be further studied in a larger cohort and in different subtypes of B-ALL. In this study, our aim was to further validate the abnormal transcript levels and investigate the potential prognostic value of RASD1 transcript levels in adult Ph-negative B-ALL patients. To our knowledge, this is the first work to study the clinical significance of RASD1 in adult Ph-negative B-ALL patients.
2. Materials and methods
2.1. Subjects and samples
We collected bone marrow samples from 92 patients with newly-diagnosed Ph-negative B-ALL diagnosed and treated in the Hematology Department of Peking University People’s Hospital, Beijing, China, between March 2009 and February 2017. Bone marrow samples from 40 healthy donors served as normal controls. The criteria for diagnosis, risk stratification and response to therapy were published previously [Citation17]. As previously reported, the patients received induction therapy of CODP ± L, also known as VDCP ± L [Citation18]. For patients who failed to achieve CR after induction therapy, revised Hyper-CVAD (B) and other regimens were administered. Ninety patients received consolidation therapy after achieving CR. Among them, 39 patients received 6–8 courses of consolidation cycles with the regimens of Hyper-CVAD (B), Hyper-CVAD (A), MTX + L-Asp or CAM, and received maintenance therapy consisting of 6-mercaptopurine and methotrexate. The detailed treatment scheme is shown in the Supplementary material. Fifty-one patients receive dallogeneic hematopoietic stem cell transplantation (allo-HSCT) from a human leukocyte antigen (HLA)-identical sibling (n = 17) or an HLA-haploidentical related donor (n = 34). The conditioning regimen for allo-HSCT and prophylaxis for graft-versus-host disease were reported previously [Citation18,Citation19]. Intrathecal methotrexate, cytarabine and dexamethasone for ≥6 doses were used for the prophylaxis of central nervous system leukemia. Patients received therapy based on doctors’ experience and patients’ intention. The subjects were followed until death, loss to follow-up or September 2018. This study was approved by the Ethics Committee of Peking University People’s Hospital, and written informed consent was obtained in compliance with the Declaration of Helsinki.
2.2. Immune phenotype, cytogenetic and molecular analyses, and minimal residual disease (MRD) detection
The immune phenotype was identified as previously described [Citation20]. The cytogenetic analysis was performed with G-banding. The molecular analyses for MLL rearrangement and IKZF1 deletion were performed as previously reported [Citation17]. A positive status of MRD was defined as the detection of over 0.1% of cells displaying leukemia-associated aberrant immune phenotypes (LAIPs) analysed by flow cytometry [Citation20]. MRD was monitored in all patients at regular intervals, including at the time of achieving CR, after every cycle of consolidation chemotherapy, and every 6 months during maintenance chemotherapy.
2.3. RNA preparation, reverse transcription and real-time quantitative PCR (RT-qPCR)
The procedures for bone marrow mononuclear cells (BMMNCs) isolation by Ficoll-Hypaque density gradient centrifugation, RNA extraction, cDNA synthesis and TaqMan-based RT-qPCR were the same as in our previous study [Citation21]. The RASD1 transcript levels were calculated as the percentage of RASD1 copies/ABL1 copies as previously described [Citation22]. ABL1 and RASD1 copy numbers were calculated from standard curves using Ct values. Samples were assayed in triplicate. The sequences of the primers and probes are shown in .
Table 1. Sequences of primers and probes used in this study.
2.4. Statistical analyses
Comparisons between two groups were performed with the Mann–Whitney U test or Student’s t-test for continuous variables and Pearson Chi-square test or Fisher’s exact test for categorical variables. To identify the optimal cut-off value that best discriminated Ph-negative B-ALL patients from healthy controls, we performed ROC analysis with values of RASD1 transcript levels of both normal control and Ph-negative B-ALL patients. The Youden Index was used to identify optimal cut-off points for RASD1 transcript levels in the diagnosis of Ph-negative B-ALL. Survival outcomes were estimated by the Kaplan-Meier method using the log-rank test. Relapse-free survival (RFS) was measured from the date of the first CR (CR1) achieved to the first relapse or death of any cause. The cumulative incidence of relapse (CIR) was calculated from the date of CR1 achieved to the date of first relapse, and death of nonrelapse was counted as competing event in our analysis. The analysis was performed with the statistical package ‘cmprsk’ in the R statistical software. Potential prognostic factors for CIR and RFS were studied with Cox proportional hazards regression and variables with P >0.1 were sequentially excluded from the model. A two-sided P <0.05 was considered significant in the analyses. Analyses were performed with SPSS software version 23.0, GraphPad Prism™ 6.01, SAS 9.4 software and the R software package.
3. Results
3.1. RASD1 transcript levels in Ph-negative B-ALL
We compared the transcript levels of RASD1 in BMMNCs from adult Ph-negative B-ALL patients at diagnosis (n = 92) and healthy controls (n = 40). The transcript levels of RASD1 in BMMNCs from patients with newly-diagnosed Ph-negative B-ALL (median 81.76%; range 0.22%-1824.52%) were significantly higher than those in healthy controls (7.59%; 0.46%-38.66%, P < 0.0001, A).
Figure 1. Transcript levels of RASD1 in B-ALL. (A) Transcript levels of RASD1 in healthy adults and adult patients with Ph-negative B-ALL. Error bars indicate the interquartile range. (B) ROC curve of transcript levels of RASD1 in Ph-negative B-ALL.
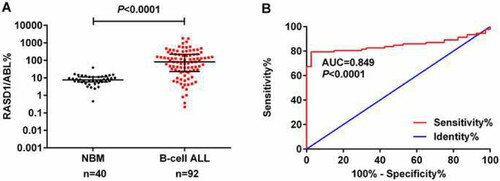
The area under the curve (AUC) was 0.849 (95% confidence interval [CI], 0.783, 0.916; P < 0.0001, B). Using the optimal cut-off value of 18.29% identified by the maximum Youden index, the rate of RASD1 overexpression in adult Ph-negative B-ALL was 77.2%. The baseline characteristics are shown in Table S1.
3.2. Low RASD1 transcript level predicts worse RFS and higher CIR in Ph-negative B-ALL
The CR rate after the first course of induction therapy was 91.3% (84/92), and the overall CR rate was 97.8% (90/92) in the cohort of 92 patients. MRD response (MRD < 0.1%) after induction was achieved in 55 of the 90 patients (61.1%) who achieved CR. Patients were divided into low- or high-RASD1 transcript level groups according to the median RASD1 transcript level (81.76%) in the cohort of patients with newly-diagnosed Ph-negative B-ALL. As shown in , clinical characteristics including gender, age, initial WBC count, bone marrow blasts, immune phenotype, IKZF1 deletion, MRD at the end of induction and treatment choice (chemotherapy vs. allo-HSCT) did not significantly differ between the groups, while a higher platelet count was found in the high-RASD1 transcript level group (P=0.039). Higher frequency of normal karyotype was identified in high-RASD1 transcript level group but MLL rearrangement was absent in this group (P = 0.021). The CR achievement after the first induction chemotherapy was similar between the low-RASD1 group and the high-RASD1 group (95.7% vs. 87.0%, P = 0.267). The MRD response (MRD < 0.1%) after induction was achieved in 30 of the 45 patients with low RASD1 transcript levels who achieved CR and 25 of the 45 patients in the high-RASD1 groups (66.7% vs.55.6%, P =0.280).
Table 2. Characteristics of patients with Ph-negative B-ALL.
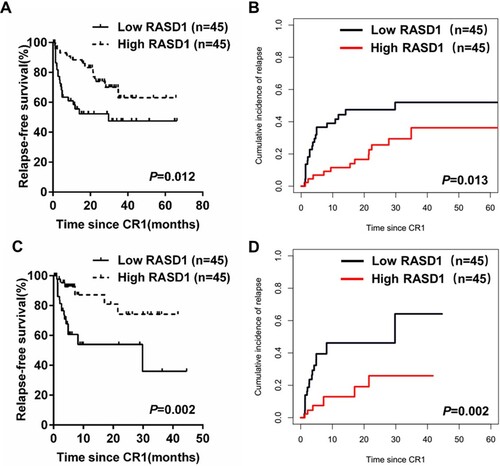
The prognostic value of the RASD1 level was studied in 90 patients achieving CR. Among the patients, the median follow-up period was 21.8 months (range 1.5–67.1 months). Kaplan-Meier analysis showed that patients with low RASD1 transcript levels had a lower 5-year RFS (47.5% [32.9%, 62.1%] vs. 63.1% [49.0%, 77.2%], P = 0.012, A) and higher 5-year CIR (52.0% [37.4%, 66.6%] vs. 36.2% [22.2%, 50.2%], P= 0.013, B) than those with high RASD1 transcript levels. Similar results were found if the patients who underwent allo-HSCT were censored at the time of HSCT (RFS: low vs. high, 35.9% [21.9%, 49.9%] vs. 74.2% [61.4%, 87.0%], P = 0.002, C; CIR: low vs. high, 64.1% [50.1%, 78.1%] vs. 25.9% [13.1%, 38.7%], P = 0.002; D).
3.3. Interaction between RASD1 transcript level and post-remission therapy
Since post-remission therapy may affect the outcomes of B-ALL patients, we analyzed the prognostic value of RASD1 transcript levels in patients receiving chemotherapy only and those who underwent allo-HSCT. Thirty-nine patients achieving CR received chemotherapy only. Patients with low RASD1 transcript levels had worse RFS (28.8% [8.4%, 49.2%] vs. 66.6% [45.9%, 87.3%], P = 0.037, A) and higher CIR (71.2% [50.8%, 91.6%] vs. 33.4% [12.7%, 54.1%], P = 0.041; B) compared with subjects with high RASD1 transcript levels.
Figure 3. RFS and CIR of high- and low-RASD1 transcript level groups of adult patients with Ph-negative B-ALL according to the post-remission therapy. (A) RFS and (B) CIR among 39 patients with Ph-negative B-ALL who received chemotherapy only. (C) RFS and (D) CIR among 45 patients with Ph-negative B-ALL who underwent allo-HSCT at CR1.
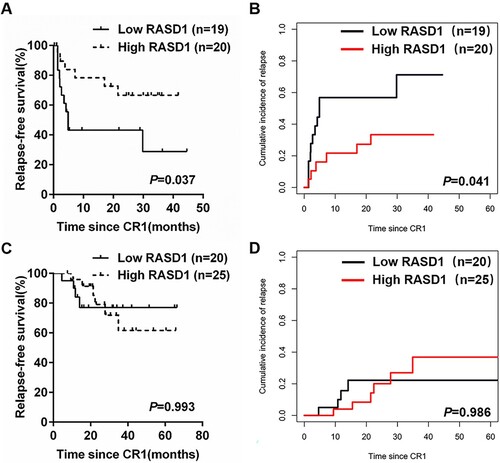
Forty-five patients received allo-HSCT at CR1. The median duration from the time of CR achievement to allo-HSCT was 4 months (1–10 months). Patients with low RASD1 transcript levels had similar RFS (77.0% [58.6%, 95.4%] vs. 61.6% [42.5%, 80.7%], P = 0.993, C) and CIR (22.2% [4.0%, 40.4%] vs. 36.8% [17.9%, 55.7%], P = 0.986; D) compared with subjects with high RASD1 transcript levels.
3.4. Multivariate analysis
To better assess the prognostic value of RASD1 transcript levels in Ph-negative B-ALL, multivariate analysis of prognostic factors was performed with a Cox regression model. In multivariate analyses including age (>35 vs. ≤35 years old), WBC count (>30 vs. ≤30×109/L), IKZF1 deletion (Yes vs. No), MRD status (≥ 0.1% vs. <0.1%), RASD1 transcript level (low vs. high) and treatment option (chemotherapy only vs. allo-HSCT), a low RASD1 transcript level, positive MRD status at the end of induction therapy and chemotherapy only were independent unfavorable prognostic factors for RFS and CIR in Ph-negative B-ALL patients (). An independent adverse impact of low RASD1 transcript levels on leukemia relapse was also observed in patients receiving chemotherapy only but not in patients who underwent allo-HSCT.
Table 3. Multivariate analyses of CIR and RFS in adults with Ph-negative B-ALL.
4. Discussion
The long-term outcomes of adult B-ALL patients remain relatively poor with high incidence of drug resistance and relapse although the remission rates reach 80%-90% due to intensified therapy regimens [Citation23]. The identification of novel molecular biomarkers may provide diagnostic and prognostic value and has been of particular interest in B-ALL. For example, high LEF1 expression was found to be associated with shorter RFS in B-ALL patients [Citation24]. High expression of BAALC was found to be predictive of chemoresistance and inferior overall survival in patients with B-ALL [Citation25]. The identification of novel molecular biomarkers is particularly imperative for patients lacking high-risk features, such as patients with Ph-negative B-ALL, and may improve risk stratification for this biologically and clinically heterogeneous subgroup. The aim of the present study was to assess the transcript level of RASD1 and to identify its value in predicting relapse in adult Ph-negative B-ALL.
RASD1 expression has been studied in multiple solid tumors. It was found to be upregulated in osteosarcoma and castrate-resistant prostate cancer [Citation8,Citation9]. RASD1 was reported to be upregulated in astrocytoma tissues and associated with good survival [Citation10]. However, RASD1 downregulation was found in renal cell carcinoma and chemotherapy-responsive oligodendroglial tumors [Citation12,Citation26]. In this study, we found that RASD1 mRNA was expressed at high levels in BMMNCs from adult Ph-negative B-ALL patients and this expression is heterogeneous, suggesting that RASD1 transcript level may have prognostic relevance. The definition of overexpression is the basis for analysing the clinical significance of RASD1 in Ph-negative B-ALL. In this study we used the median transcript level of RASD1 in Ph-negative B-ALL patients to divide the patients into a high expression group and a low expression group as reported [Citation20,Citation27]. We found that a low RASD1 transcript level at diagnosis was associated with a higher risk of relapse in patients with Ph-negative B-ALL, especially in patients receiving chemotherapy only. However, the outcomes for CIR and RFS did not differ significantly according to RASD1 transcript levels in patients who underwent allo-HSCT, suggesting that allo-HSCT may overcome the adverse impact of lower RASD1 transcript levels on relapse. However, this correlation should be interpreted with caution, because it may be partially due to the relatively small number of cases in the allo-HSCT group. In addition, many factors may affect the outcome of allo-HSCT, such as donor-recipient gender matching, HLA disparity, and monitoring and intervention for MRD [Citation20]. An analysis combining RASD1 transcript levels and the known factors in a large cohort may achieve a better understanding of the prognostic value of RASD1 in B-ALL.
It has been reported that RASD1 played a role in regulating the development of tumors as a negative RAS signaling regulating gene [Citation28]. RASD1 overexpression was reported to suppress cell growth in breast cancer and lung carcinoma cells, inhibit migration and invasion of glioma cells and participate in induced-apoptosis in breast cancer and prostate cancer cells [Citation10,Citation11,Citation13,Citation14,Citation29,Citation30]. In B lymphocytes, RASD1 has been reported to serve as a potential regulatory protein and high levels of RASD1 mRNA were associated with low proliferation of B cells [Citation15]. This might explain why patients with high RASD1 levels showed better outcomes. However, a recent study showed that a high RASD1 transcript level (>0.665%) predicted poor overall survival in patients with B-ALL and promoted chemotherapy resistance in B-ALL cells [Citation16]. In that study the cut-off value of RASD1 was identified with ROC analysis based on patient death, which might not be suitable for the analysis of relapse. The difference in patient subgroups, the detection methods and identification of cut-off values, and possible bias caused by the limited number of cases between the two studies might explain the inconsistency. Besides, the 17p (TP53) deletion might occur in B-ALL and might affect the expression of RASD1. Since no 17p deletion was found by karyotype analysis in our cohort, the correlation between 17p deletion and RASD1 expression should be studied in the future. Therefore, the biological function of RASD1 should be further clarified both in vitro and in vivo.
The analysis of RASD1 transcript levels in the prognosis of B-ALL patients was complicated due to confounding factors. Therefore, we performed multivariate analysis to resolve these confounding factors. Studies have shown that the prognostic impact of traditional factors such as age and WBC count may be outweighed by that of cytogenetic and molecular features [Citation4]. The prognostic value of IKZF1 deletion was not significant in this study, probably because there were only 28 patients with IKZF1 deletion, which was too few in each group to provide a significant prognostic value. Furthermore, the prognostic value of IKZF1 deletion was more significant in patients with Ph-positive or Ph-like ALL, while our study mainly focused on patients with Ph-negative B-ALL and the Ph-like status was not examined in our cohort. The MRD response status did not differ between high- and low-RASD1 transcript level groups, but an effect on relapse was still retained. This might suggest that RASD1 did not affect leukemia remission, which was consistent with the similar CR rates in the high- and low-RASD1 transcript level groups. Independent from the post-treatment markers like MRD, RASD1 transcript levels at diagnosis might be helpful for stratification of patients with Ph-negative B-ALL into risk-adapted therapy. One limitation of our multivariate analysis was that we did not include the potential prognostic variable, MLL rearrangement, because too few subjects had this alteration in each group. Therefore, the prognostic significance of RASD1 transcript levels in B-ALL should be further confirmed in a larger, prospective and randomized cohort.
In summary, our results showed that a low RASD1 transcript level was an independent risk factor for relapse in patients with Ph-negative B-ALL, particularly in patients receiving chemotherapy only. Further cohort validation and study of the molecular mechanisms of RASD1 may provide us with better understanding of its prognostic value in Ph-negative B-ALL.
Supplemental Material
Download PDF (99.8 KB)Acknowledgements
We would like to thank all our colleagues at Peking University Institute of Hematology for their cooperation in sample collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jamrog L, Chemin G, Fregona V, et al. PAX5-ELN oncoprotein promotes multistep B-cell acute lymphoblastic leukemia in mice. Proc Natl Acad Sci U S A. 2018;115(41):10357–10362.
- Nagafuji K, Miyamoto T, Eto T, et al. Prospective evaluation of minimal residual disease (MRD) monitoring to predict prognosis of adult patients with Ph-negative ALL. Eur J Haematol. 2019;103(3):164–171.
- Wolach O, Amitai I, DeAngelo D J. Current challenges and opportunities in treating adult patients with Philadelphia-negative acute lymphoblastic leukaemia. Br J Haematol. 2017;179(5):705–723.
- Jacobson S, Tedder M, Eggert J. Adult acute lymphoblastic leukemia: A genetic overview and application to clinical practice. Clin J Oncol Nurs. 2016;20(6):E147–E154.
- Tu Y, Wu C. Cloning, expression and characterization of a novel human Ras-related protein that is regulated by glucocorticoid hormone. Biochim Biophys Acta. 1999;1489(2-3):452–456.
- Cha J Y, Kim H J, Yu J H, et al. Dexras1 mediates glucocorticoid-associated adipogenesis and diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(51):20575–20580.
- Carlson G C, Lin R E, Chen Y, et al. Dexras1 a unique ras-GTPase interacts with NMDA receptor activity and provides a novel dissociation between anxiety, working memory and sensory gating. Neuroscience. 2016;322:408–415.
- Joeri B, Thijs W, Johannes B, et al. Identification of novel candidate oncogenes in chromosome region 17p11.2-p12 in human osteosarcoma. Plos One. 2012;7(1):e30907.
- O'Neill D, Jones D, Wade M, et al. Development and exploitation of a novel mutant androgen receptor modelling strategy to identify new targets for advanced prostate cancer therapy. Oncotarget. 2015;6(28):26029–26040
- Gao S, Jin L, Liu G, et al. Overexpression of RASD1 inhibits glioma cell migration/invasion and inactivates the AKT/mTOR signaling pathway. Sci Rep. 2017;7(1):3202.
- Vaidyanathan G, Cismowski M J, Wang G, et al. The Ras-related protein AGS1/RASD1 suppresses cell growth. Oncogene. 2004;23(34):5858.
- Dalgin G S, Holloway D T, Liou L S, et al. Identification and characterization of renal cell carcinoma gene markers. Cancer Inform. 2007;3(3):65.
- Tian J, Duan Y X, Bei C Y, et al. Calycosin induces apoptosis by upregulation of RASD1 in human breast cancer cells MCF-7. Horm Metab Res. 2013;45(8):593–598.
- Liu X, Li Y, Chen Q, et al. Up-regulating of RASD1 and apoptosis of DU-145 human prostate cancer cells induced by Formononetin in vitro. Asian Pac J Cancer Prev. 2014;15(6):2835–2839.
- Lindsey J. Dexamethasone-induced Ras-related protein 1 is a potential regulatory protein in B lymphocytes. Int Immunol. 2007;19(5):583–590.
- Wang S, Wang C, Wang W, et al. High RASD1 transcript levels at diagnosis predicted poor survival in adult B-cell acute lymphoblastic leukemia patients. Leuk Res. 2019;80:26–32.
- Yao Q M, Liu K Y, Gale R P, et al. Prognostic impact of IKZF1 deletion in adults with common B-cell acute lymphoblastic leukemia. BMC Cancer. 2016;16:269.
- Yan C H, Jiang Q, Wang J, et al. Superior survival of unmanipulated haploidentical hematopoietic stem cell transplantation compared with chemotherapy alone used as post-remission therapy in adults with standard-risk acute lymphoblastic leukemia in first complete remission. Biol Blood Marrow Transplant. 2014;20(9):1314–1321.
- Yan C H, Liu D H, Liu K Y, et al. Risk stratificationdirected donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012;119(14):3256–3262.
- Zhang J, Lu W Y, Zhang J M, et al. S100a16 suppresses the growth and survival of leukaemia cells and correlates with relapse and relapse free survival in adults with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia. Br J Haematol. 2019;185:836–851.
- Ruan G R, Qin Y Z, Chen S S, et al. Abnormal expression of the programmed cell death 5 gene in acute and chronic myeloid leukemia. Leuk Res. 2006;30(9):1159–1165.
- Beillard E, Pallisgaard N, van der Velden V H, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17(12):2474–2486.
- Jabbour E, O'Brien S, Konopleva M, et al. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–2528.
- Kuhnl A, Gokbuget N, Kaiser M, et al. Overexpression of LEF1 predicts unfavorable outcome in adult patients with B-precursor acute lymphoblastic leukemia. Blood. 2011;118(24):6362–6367.
- Kuhnl A, Gokbuget N, Stroux A, et al. High BAALC expression predicts chemoresistance in adult B-precursor acute lymphoblastic leukemia. Blood. 2010;115(18):3737–3744.
- Shaw E J, Haylock B, Husband D, et al. Gene expression in oligodendroglial tumors. Cell Oncol. 2011;34(4):355–367.
- Wang S J, Wang P Z, Gale R P, et al. Cysteine and glycine-rich protein 2 (CSRP2) transcript levels correlate with leukemia relapse and leukemia-free survival in adults with B-cell acute lymphoblastic leukemia and normal cytogenetics. Oncotarget. 2017;8(22):35984–36000.
- Wong K Y, Yao Q, Yuan L Q, et al. Frequent functional activation of RAS signalling not explained by RAS/RAF mutations in relapsed/refractory multiple myeloma. Sci Rep. 2018;8(1):13522.
- de Souza Rocha Simonini P, Breiling A, Gupta N, et al. Epigenetically deregulated microRNA-375 Is involved in a positive Feedback Loop with Estrogen Receptor in breast cancer cells. Cancer Res. 2010;70(22):9175–9184.
- Munagala R, Aqil F, Vadhanam MV, et al. MicroRNA ‘signature’ during estrogen-mediated mammary carcinogenesis and its reversal by ellagic acid intervention. Cancer Lett. 2013;339(2):175–184.