ABSTRACT
Objectives
The remarkable effect of arsenic trioxide (ATO) was verified, but elevated gamma-glutamyltransferase (GGT), aminotransferases (ALT and AST) are generally observed in acute promyelocytic leukemia (APL) patients undergoing ATO treatment. However, utilization of hepatoprotective agents or discontinuation of ATO may inhibit ATO efficacy. In order to maintain ATO effect from hepatoprotective agents’ influence so we investigate relationships between single elevation in GGT and hepatocellular injury in this study.
Methods
Correlation of GGT variation and leukocyte counts were analyzed in all 81 APL patients, correlations among liver enzymes (ALT, AST and GGT) were also analyzed in patients without prophylactic hepatoprotective agents. In following study, we take the clinical observation of changes in aminotransferases in patients with single elevation in GGT without hepatoprotective agents.
Results
The average elevated GGT in the WBC abnormal group was more than the normal group (53.86U/L vs. 31.03U/L, P = 0.008), a positive Pearson’s correlation of GGT variation and changed leukocyte counts in patients without prophylactic hepatoprotective agents. There are no significant correlation between aminotransferases (ALT and AST) and GGT but correlation between ALT and AST was statistically significant (R = 0.649, P = 0.000). For APL patients with single elevation in GGT, ALT and AST levels were normal throughout the ATO treatment without hepatoprotective agents.
Conclusion
Single elevation in GGT without elevated aminotransferases can’t be identified as hepatotoxicity, and the elevated levels of GGT are associated with increasing leukocyte counts. Continue single-agent ATO without prophylactic hepatoprotective agents is recommended in APL patients with single elevation in GGT, in order to maintain ATO effect.
Introduction
Arsenic trioxide (As2O3, ATO) was firstly used for the treatment of acute promyelocytic leukemia (APL) patients in the First Affiliated Hospital, Harbin Medical University in 1970s. ATO is a striking medication for patients with APL, even in relapsed APL patients, more than 90% achieved complete remission (CR) [Citation1,Citation2]. However, ATO has some toxic effects and is accompanied by a serious of side effects in a small number of patients [Citation3–5]. Concerns have been raised about the hepatotoxicity in APL patients treated with ATO [Citation6]. Elevated gamma-glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST) are generally observed. Hepatotoxicity was observed in 65.5% of APL patients treated with single-agent ATO, especially during the induction phase. Increased liver enzymes levels were the major manifestations of hepatotoxicity [Citation6,Citation7]. It has been reported that ALT and AST in the early stage of ATO treatment were significantly higher than the beginning, but the elevated values dramatic decline following the treatment [Citation6]. Generally, we thought that changes in ALT and AST were indicators of liver injury [Citation8]. But, GGT is not considered as main indicator of liver injury. GGT may be associated with WBC [Citation9–11]. Whether definite relations existed among GGT, ALT and AST?
Prophylactic application of hepatoprotective agents is easy to inhibit ATO efficacy [Citation12]. Thus, how to reduce hepatotoxicity without inhibiting ATO efficacy is an impending problem. At present, clinical manifestations and laboratory test are major indicators for monitoring ATO hepatotoxicity. When the abnormalities appeared, patients have suffered side effects. The hepatotoxicity assessment studies not yet seen and even basic researches are rarely reported. In our study, we tried to explore predictors that contributed to hepatotoxicity comprehensive analysis in order to reduce unnecessary prophylactic use of hepatoprotective agents and maintain ATO effect and guide the individualized application of ATO in the future through our researches.
Materials and methods
ATO treatment Protocol
The ATO solution (10 mg/10 mL) was supplied by Harbin Yida Pharmaceutical Company, dissolved in 500 mL 5% dextrose and administered daily at a dose of 0.20 mg/kg for children ≤ 6 years old and 0.16 mg/kg for children >6 years old, with a maximum daily dose of 10 mg. The total ATO dose was infused intravenously over the course >18 h.
Patients received arsenic trioxide as the induction regimen, other medications during the ATO treatment were only for reduce hyperleukocytosis and prevent the emergence of a life-threatening differentiation syndrome, mostly using hydroxyurea and (or) short-term low-dose anthracycline chemotherapy drugs (43.21%), of which using hydroxyurea alone accounts for 17.28%.
In the induction therapy stage, each patient would manage to build regular lifestyles and take exercise with low intensity, such as walk and yoga. Seafood consumption was prohibited in order not to affect arsenical concentration during the treatment progress.
Liver function
All biochemical parameters in the retrospective study are based on reports from biochemical laboratory of laboratory department by Beckman Coulter AU5800 series automatic biochemical analyzer. Liver function was monitored weekly. Patients with liver impairment, comprehensive monitoring and the necessary supportive therapy were provided until the end of the treatment and patients achieved CR. During ATO treatments, patients with abnormal liver enzymes ALT, AST or GGT were defined as having impaired liver function (absolute values of ALT>40U/L; AST>40U/L; GGT>50U/L).△ALT, △AST and △GGT as changes in liver enzymes between the time of the first occurrence of liver impairment and the initiation of ATO treatment.
Patients and study groups
All 81 APL patients were admitted to the Department of Hematology, First Affiliated Hospital, Harbin Medical University, from February 2011 to April 2018. The inclusion criterion was newly diagnosed APL patients with normal liver enzymes and then followed abnormal liver function. Exclusion criteria included previous history of hepatitis, excessive drinking and other diseases of hepatobiliary and abnormal renal function, liver function or electrocardiographic findings.
Retrospective study: ①subjects were divided into the ‘higher’ or ‘lower’ elevated GGT(ΔGGT) groups based on △GGT between initial treatment and the first liver impairment occurred. Average elevated White blood cell (ΔWBC) counts were compared between the two groups.② subjects were divided into the ‘higher’ or ‘lower’ elevated WBC (ΔWBC) groups based on ΔWBC between initial treatment and the first liver impairment occurred. Average elevated GGT (ΔGGT) were compared between the two groups.③ subjects were divided into the ‘abnormal’ or ‘normal’ GGT groups based on GGT when the first liver impairment occurred. Average elevated WBC (ΔWBC) counts were compared between the two groups.④ subjects were divided into the ‘abnormal’ or ‘normal’ WBC groups based on WBC when the first liver impairment occurred. Average elevated GGT (ΔGGT) were compared between the two groups. (2) Prospective study: For patients with elevated ALT and/or AST we undertook various measures according to the different clinical symptoms; for patients with only elevated GGT, no hepatoprotective agents were used in the patients and monitoring liver function.
Statistical analysis
All data were analyzed using SPSS 17.0 software and Graph Pad Prism 6.0. A Pearson's correlation coefficient analysis was applied to assess correlations among variations in liver enzymes (ΔALT, ΔAST and ΔGGT) or correlation of elevated leukocyte and ΔGGT between the initiation of ATO treatment and the first occurrence of liver impairment; The Wilcoxon–Mann–Whitney test was used to compare elevated WBC or elevated GGT in different groups. All tests were two-sided, and a P-value of less than 0.05 was considered statistically significant.
Results
Patients’ clinical characteristics and physiological complications
All patients’ clinical characteristics were as follows (): Physiological complication associated with ATO treatment were nausea, vomiting, fever, and infection in this retrospective study. These physiological complications are with a controllable range, and are all reversed by relevant symptomatic treatments.
Table 1. Clinical characteristics of study patients.
GGT variation and leukocyte counts
We divided all 81 patients into two groups according to elevated GGT(ΔGGT) level between initial treatment and the first liver impairment occurred ((A)) and compared the elevated WBC(ΔWBC) in these two groups. The average ΔWBC in the lower group (40 patients) was 4.41 × 109/L, and in the higher group (41 patients), it was 8.74 × 109/L.
Figure 1. (A) Elevated WBC values between initial treatment and the first liver impairment occurred; (B) elevated GGT level between initial treatment and the first liver impairment occurred.
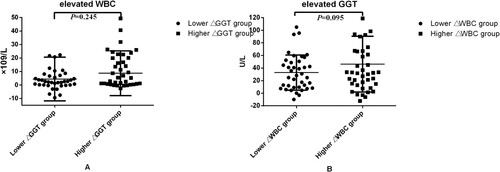
The 81 included patients who were divided into two groups, lower ΔWBC group (40 patients) and higher △WBC group (41 patients) based on the ΔWBC values between initial treatment and the first liver impairment occurred ((B)). The average ΔGGT in the lower group was 32.74 U/L, and in the higher group, it was 47.05 U/L.
The 81 patients were divided into GGT normal group and GGT abnormal group, based on the GGT values when the liver impairment first occurred ((A)). The average ΔWBC in the GGT abnormal group (46 patients) was 9.51 × 109/L, and in the normal group (35 patients), it was 2.56 × 109/L, the results show that changes in WBCs in the two groups were difference (P = 0.064).
Figure 2. (A) Elevated WBC values between initial treatment and the first liver impairment occurred; (B) elevated GGT level between initial treatment and the first liver impairment occurred.
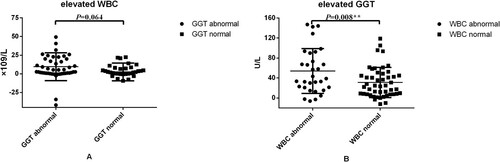
All 81 patients were divided into WBC normal group and WBC abnormal group, based on the WBC values when the liver impairment first occurred ((B)). The average ΔGGT in the WBC abnormal group (33 patients) was 53.86 U/L, and it was 31.03 U/L in the normal group (48 patients), it’s a statistically significant difference.
We also analyzed the correlation of GGT variation and changed leukocyte counts, there were statistically significant correlation in ΔGGT and ΔWBC(R = 0.258 P = 0.022*).
Correlations among ALT, AST and GGT levels
We analyzed relationship of GGT and WBC in all patients. In the following analysis, we analyzed relationships among liver enzymes in 46 APL patients without prophylactic hepatoprotective agents, because hepatoprotective agents affect ALT and AST level.
In all 46 patients without prophylactic hepatoprotective agents, correlations of changes in liver enzyme values (ΔALT, ΔAST and ΔGGT) between the initiation of ATO treatment and the first occurrence of liver impairment were analyzed (). The correlation between ΔALT and ΔAST (R = 0.649) was statistically significant (P = 0.000)(R > 0.5 is considered a high correlation). Additionally, we found no significant correlation between ΔALT or ΔAST and ΔGGT, respectively.
Table 2. Analysis of correlations between variations in enzymes levels.
Management of liver impairment
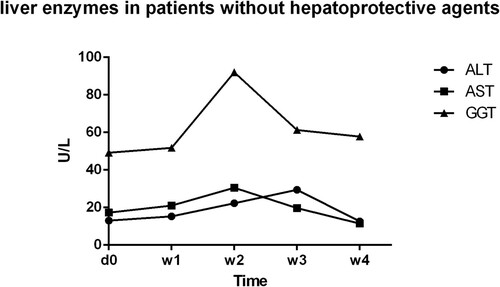
In retrospective study, the inclusion criterion of all 81 patients was newly diagnosed APL patients with normal liver enzymes. A totally five time points are selected to show the level of ALT, AST & GGT at the initiation of ATO treatment, during the treatment, and after the withdrawal of ATO treatment (). Patients with elevated transaminases (ALT and AST) received hepatoprotective agents, such as glutathione, ammonium glycyrrhizinate S, diisopropylamine dichloroacetate, magnesium isoglycyrrhizinate, polygene phosphatidylcholine and bicyclol. At the end of the treatment, liver enzymes (ALT and AST) of most patients returned to normal levels. In prospective study, no hepatoprotective agents and no temporary discontinuation of ATO were used in the 12 patients whose ALT and AST level were normal in the treatment process, no matter GGT levels were abnormal or not (). Moreover, none of the related clinical manifestations and side effects impeded the therapeutic treatment of these 12 APL patients. Under continuous observation for hepatotoxicity, the ALT and AST levels of these 6 patients with single elevation of GGT remained normal; GGT levels decreased in the patients. In brief, ALT and AST levels were normal throughout the ATO treatment without the use of hepatoprotective agents, regardless of the elevated GGT level.
Figure 3. Liver enzymes in all patients: liver enzymes’ variation tendency in all patients. Hepatoprotective agents were used in some patients when liver enzymes were abnormal during ATO treatment process. initiation, the initiation of ATO treatment; W1,one week after ATO treatment; W2, two weeks after ATO treatment; W3, three weeks after ATO treatment; W4, four weeks after ATO treatment; withdrawal, withdrawal of ATO treatment and the last biochemical parameters measurement during the treatment.
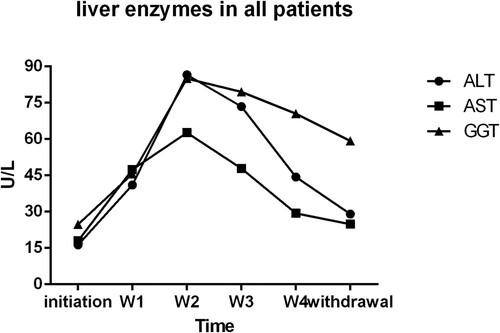
Figure 4. Liver enzymes in patients without hepatoprotective agents: liver enzymes’ variation tendency in patients whose ALT and AST level were normal during the treatment process, no matter GGT levels were abnormal or not; d0,before ATO treatment; W1,one week after ATO treatment; W2, two weeks after ATO treatment; W3, three weeks after ATO treatment; W4, four weeks after ATO treatment.
Discussion
The remarkable effect of ATO was verified in APL patients, but the change of liver function is common. Increased ALT and AST are considered as indicators of hepatotoxicity, how about elevated GGT? Previous researches indicated hepatoprotective agents and temporary discontinuation of ATO are generally administered to patients in order to reverse the elevated liver enzymes. However, these measures will lead to arsenic concentration lower than the apoptosis concentrations, following a series of side effects. Because of the inhibitory effect of hepatoprotective agents in ATO, it’s better not to prophylactic use hepatoprotective agents as much as possible.
GGT is a glutamyl Trans peptidase that catalyses the transmembrance transport of gamma-glutamyl to exogenous amino acids, glutathione (GSH) or water [Citation13]. In clinical settings, elevated GGT is generally observed in patients with cholecystitis and cholelithiasis [Citation14]. Some researchers found that GGT was elevated in leukemia cells and significantly decreased after chemotherapy [Citation9,Citation10,Citation15]. Rzymowska showed that GGT activity and levels were higher in patients with AML than in ALL [Citation16]. These findings suggest that leukocyte counts associated with these changes. So, we analyzed relationship between WBC and GGT in all 81 APL patients. △GGT was high in higher △WBC group, in terms of, elevated GGT was affected by elevated WBC counts. Increased GGT levels may be a protective factor during leukemia therapy.
Some researchers found that GGT activity and the mRNA level of GGT were elevated in cells treated with arsenicals [Citation17,Citation18] and hepatoprotective agents [Citation19]. In addition, a complementary increase in GGT level was also observed in the gamma-glutamyl cycle for APL patients administered hepatoprotective agents.
Under the excluded patients with prophylactic application of hepatoprotective agents, we analyzed correlations among liver enzymes in our clinical center. Correlations among △ALT, △AST and △GGT were analyzed between the first occurrence of liver impairment and the initiation of ATO treatment, this time point facing application of hepatoprotective agents or not. Since the initial values of ALT and AST are different, therefore we couldn’t evaluate liver impairment just rely on absolute values of ALT and AST to evaluate liver damage.
In previous studies, correlations of liver injury and variation in liver enzyme levels at different time points were not confirmed [Citation20]. The results revealed that GGT levels were not significantly associated with ALT and AST levels in APL patients treated with ATO as a single agent, but there was a close correlation between ALT and AST levels(R = 0.649, P ≤ 0.000). Based on the above statistical analysis, we can thought there are maybe different mechanisms of elevated GGT from changes in ALT and AST which is our future study.
γ-Glutamyl cycle is a glutathione resynthesize reaction in cells. The key enzyme of the cycle is gamma-glutamyltransferase which its localization is in the cell membrane. The toxicity of drugs to cells mainly depends on intracellular sulfhydryl levels, and glutathione is the main component. On normal physiological conditions, GSH levels of intracellular and extracellular are effectively regulated by γ-glutamyl cycle in U937 cell line. The GSH in the outside of the cell couldn’t be transferred into the cell, but is re-synthesized in the cell after the hydrolysis of the GGT [Citation13,Citation21]. Previous studies have demonstrated that the elevated GGT in a variety of tumor cells may increase the transport of GSH to cells, accompanied by the increase of intracellular GSH level [Citation22]. Our study indirectly confirmed that the increase in GGT may be due to the enhancement of the γ-glutamyl cycle of the cell membrane which is induced by arsenicals, not as a result of hepatocyte injury.
In addition, a complementary increase in GGT level was observed in the gamma-glutamyl cycle for APL patients administered hepatoprotective agents. GSH and cysteine, major hepatoprotective agents, are also important substrates of GGT [Citation23]. Therefore, metabolic interactions and mutual transformation occurs among most hepatoprotective agents. These hepatoprotective agents include sulfhydryl (GSH), methyl donor (methionine) and glycyrrhizinate (magnesium isoglycyrrhizinate) compounds. The metabolism of these drugs enhances the γ-glutamyl cycle, which indicates that GSH metabolism is active.
The bile duct injury may result in an increase in GGT [Citation24], but the occurrence of the side effect of bile duct injury is not appeared in the APL patients treated with the ATO in literature and clinic [Citation25].
In our manuscript, we emphasized that ‘hepatotoxicity' is not the only cause of elevated GGT level, and we then proposed the ‘delay or withdraw the application of hepatoprotective agents'. We also did not try to exclude any of these possibilities, and we advocate narrowing the scope of hepatoprotective agent application.
Based on the above theory, in our study, some APL patients with an increase in GGT level alone without any hepatoprotective intervention, ALT and AST levels were always normal in treatment process. Hence, we hypothesized that an isolated increase in GGT level is not solely the result of hepatocellular injury during ATO treatment of APL.
In addition, there are different understandings in prophylactic application of hepatoprotective agents [Citation26] that they are not recommended in any APL treatment guidelines to date. Meanwhile, we hope to change the situation according to our research that the physicians routinely applied liver protective agents for APL patients.
As described above, the initial values of ALT and AST are different, therefore we could not evaluate liver impairment just rely on absolute values of ALT and AST to evaluate liver damage. In our study, we carried out dynamic data analysis: changes in ALT and AST one week treatment latter and elevated peak values. The first week of ATO treatment is a critical period, so we focus on the first week of liver function changes in order to rule out any interference in ATO effect.
Our research firstly studied correlated relationships among elevated liver enzymes in the APL patients undergoing single-agent ATO treatment. Our study focused on the critical period in early ATO treatment stage, which is different from the focus of previous reports.
Therefore, for APL patients with single elevation of GGT, close monitoring of liver function suggested, instead of prophylactic applying hepatoprotective intervention, in order to maintain ATO efficacy.
Ethics statement
This study was approved by Medical Ethics Committee of the First Affiliated Hospital of Harbin Medical University. Written informed consent was obtained from all subjects.
Acknowledgements
The authors gratefully acknowledge all teachers in Central Laboratory, First Affiliated Hospital of Harbin Medical University for the continuous generous support of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lengfelder E, Lo-Coco F, Ades L, et al. Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European leukemia Net. Leukemia. 2015;29:1084–1091.
- Lou Y, Ma Y, Sun J, et al. Evaluating frequency of PML-RARA mutations and conferring resistance to arsenic trioxide-based therapy in relapsed acute promyelocytic leukemia patients. Ann Hematol. 2015;94(11):1829–1837.
- Kuo YJ, Liu YJ, Way TD, et al. Synergistic inhibition of leukemia WEHI-3 cell growth by arsenic trioxide and Hedyotis diffusa Willd extract in vitro and in vivo. ExpTher Med. 2017;13(6):3388–3396.
- De Thé H, Le Bras M, Lallemand-Breitenbach V. The cell biology of disease: acute promyelocytic leukemia, arsenic, and PML bodies. Cell Biol. 2012;198:11–21.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121.
- Mathews V, Desire S, George B, et al. Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia. 2006;20:881–883.
- Hao L, Zhao J, Wang X, et al. Hepatotoxicity from arsenic trioxide for pediatric acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2013;35:e67–e70.
- Kim WR, Flamm SL, Di Bisceglie AM, et al. Public Policy Committee of the American Association for the Study of Liver Disease: Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363–1370.
- Traverso N, Ricciarelli R, Nitti M, et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev. 2013;2013:972913. DOI: https://doi.org/10.1155/2013/972913.
- Täger M, Ittenson A, Franke A, et al. gamma-Glutamyl trans peptidase-cellular expression in populations of normal human mononuclear cells and patients suffering from leukemias. Ann Hematol. 1995;70(5):237–242.
- Ishizuka Y, Moriwaki S, Kawahara-Hanaoka M, et al. Treatment with anti-gamma-glutamyl trans peptidase antibody attenuates osteolysis in collagen-induced arthritis mice. J Bone Miner Res. 2007;22(12):1933–1942.
- Sui M, Zhang Z, Zhou J. Inhibition factors of arsenic trioxide therapeutic effects in patients with acute promyelocytic leukemia. Chin Med J (Engl). 2014;127(19):3503–3506.
- Djavaheri-Mergny M, Accaoui MJ, Rouillard D, et al. Gamma-glutamyl trans peptidase activity mediates NF-kappa B activation through lipid peroxidation in human leukemia U937 cells. Mol Cell Biochem. 2002;232(1–2):103–111.
- Peng WK, Sheikh Z, Paterson-Brown S, et al. Role of liver function tests in predicting common bile duct stones in acute calculus cholecystitis. Br J Surg. 2005;92:1241–1247.
- Shizuka Y, Moriwaki S, Kawahara-Hanaoka M, et al. Treatment with anti-gamma-glutamyl trans peptidase antibody attenuates osteolysis in collagen-induced arthritis mice. J Bone Miner Res. 2007;22:1933–1942.
- Rzymowska J. Activities of enzyme transducing extracellular signals–gamma glutamyltransferase and enzymes metabolizing glutathione in acute lymphoblastic and myeloid human leukemias. Neoplasma. 1995;42:53–56.
- Cheng YH, Ou BR, Cheng LC, et al. Glutathione regulation in arsenic-induced porcine aortic endothelial cells. Toxicol in Vitro. 2008;22:1832–1839.
- Giommarelli C, Corti A, Supino R, et al. Gamma-glutamyltransferase-dependent resistance to arsenic trioxide in melanoma cells and cellular sensitization by ascorbic acid. Free Radic Biol Med. 2009;46:1516–1526.
- Soria EA, Eynard AR, Quiroga PL, et al. Differential effects of quercetin and silymarin on arsenite-induced cytotoxicity in two human breast adenocarcinoma cell lines. Life Sci. 2007;81:1397–1402.
- Chen L, Wang J, Hu X, et al. Meta-analysis of all-trans retinoic acid-linked arsenic trioxide treatment for acute promyelocytic leukemia. Hematology. 2014;19:202–207.
- Kwiecień I, Iciek M, Włodek L. Acceleration of anaerobic cysteine transformations to sulfane sulfur consequent to γ-glutamyl transpeptidase inhibition. Sci World J. 2012;2012:253724. DOI: https://doi.org/10.1100/2012/253724.
- Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res. 2014;122:103–141. DOI: https://doi.org/10.1016/B978-0-12-420117-0.00003-7.
- Khurana H, Meena VK, Prakash S, et al. Preclinical evaluation of a potential GSH ester based PET/SPECT imaging probe DT(GSHMe)₂ to detect gamma glutamyl transferase over expressing tumors. PLoS One. 2015;10:e013428.
- Westerkamp AC, Mahboub P, Meyer SL, et al. End-ischemic machine perfusion reduces bile duct injury in donation after circulatory death rat donor livers independent of the machine perfusion temperature. Liver Transpl. 2015;21(10):1300–1311.
- Ling S, Xie H, Yang F, et al. Metformin potentiates the effect of arsenic trioxide suppressing intrahepatic cholangiocarcinoma: roles of p38 MAPK, ERK3, and mTORC1. J Hematol Oncol. 2017;10 (1):59. DOI: https://doi.org/10.1186/s13045-017-0424-0.
- Yang D, Lv Z, Zhang H, et al. Activation of the Nrf2 signaling pathway involving KLF9 Plays a critical role in Allicin resisting against arsenic trioxide-induced hepatotoxicity in rats. Biol Trace Elem Res. 2017;176(1):192–200.
