ABSTRACT
Objectives
To analyze the outcomes of patients who received autologous stem cell transplantation (auto-SCT), matched sibling donor stem cell transplantation (MSD-SCT) and haploidentical stem cell transplantation (haplo-SCT) and provide the basis for the choice of transplantation method in Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).
Methods
We retrospectively investigated the outcomes of 119 adult patients with Ph+ ALL in our center. The overall survival (OS) rate, leukemia-free survival (LFS) rate, cumulative incidence of relapse (CIR) rate, non-relapse mortality (NRM) rate and the impact of achievement of complete molecular response (CMR) within 3 months and sustaining CMR up to transplantation (s3CMR) on transplantation method were explored.
Results
The estimated OS, LFS, CIR and NRM rates at 3 years were not significantly different among three groups (p = 0.960, 0.917, 0.375 and 0.096, respectively). For the 65 patients who achieved s3CMR, there was no significant difference in OS (84.5% vs 72.5% vs 100%, p = 0.374), LFS (75.2% vs 64.5% vs 83.3%, p = 0.668), CIR (17.2% vs 8.1% vs 16.7%, p = 0.583) and NRM (3.1% vs 23.4% vs 0%, p = 0.055) among auto-SCT group, MSD-SCT group and haplo-SCT group. However, in patients who did not achieve s3CMR, auto-SCT recipients tended to have higher CIR (60% vs 33.2% vs 24.0%, p = 0.013) than the allo-HSCT group.
Conclusions
Auto-SCT with maintenance therapy after HSCT appears to be an attractive treatment option for patients with Ph+ ALL especially for those whose s3CMR was kept up to transplantation. For non-s3CMR patients, allogeneic transplantation may be more effective from lower relapse.
Introduction
Adult Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is a high-risk subtype of acute lymphoblastic leukemia with lower complete remission and higher relapse rates [Citation1,Citation2]. After the incorporation of TKIs into chemotherapy, long-term survival rates increased to 30–80% [Citation3]. However, allo-HSCT is still the standard consolidation therapy for patients with available donors [Citation4–6]. The efficacy of matched related donor and matched unrelated donor SCT (URD-SCT) has been well confirmed. An analysis from southwest China also indicated a favorable outcome of haplo-HSCT in Ph+ ALL [Citation7]. In the TKI era, auto-SCT also can be used for eligible patients. TKIs can effectively reduce the tumor burden and result in negative or low level minimal residual disease (MRD) before auto-SCT, thus the absence of graft-versus-leukemia effect and the potential contamination of leukemic cells in the graft pose less concerns [Citation8]. An analysis by the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT) showed that myeloablative auto-SCT and allo-SCT demonstrated comparable outcomes for patients with Ph+ ALL in first CMR [Citation9]. In the present study, we retrospectively analyzed the outcomes of patients who received auto-SCT, MSD-SCT, and haplo-SCT to provide the basis for better selection of treatment.
Methods
Study design and data collection
In this investigation, we retrospectively collected clinical data from medical documents in our center. The eligible candidates must comply the following criteria: (1) age between 15 and –65 years, (2) diagnosis of Ph+ ALL according to WHO criteria, (3) treatment with TKIs before SCT, (4) CR at transplantation, (5) auto, MSD, or haplo-SCT performed between January 2008 and October 2019, (6) myeloablative conditioning. MSD-SCT was preferred to patients who had HLA-identical siblings. Auto-SCT was employed as an alternative strategy for patients who lack a related or unrelated donor with an identical HLA type or haploid donor. The study was approved by the ethics committees according to the guidelines of the Declaration of Helsinki. All patients provided informed consent before participating in the study.
Sequencing method
DNA extracted from bone marrow or blood samples was genotyped with Ion Ampliseq next-generation sequencing approach to target 112 genes related to hematological malignancies. All detected genes (exon regions) were screened by the Thousands Genome Project, COSMIC (Somatic Mutation Directory in Cancer) and PolyPhen-2 (Polymorphism Phenotyping 2) database.
Chemotherapy with TKIs prior to transplantation
Patients newly diagnosed in our center were given a standard induction regimen VDCP (vincristine 1.4 mg·m−2·d−1, maximum 2 mg/d, days 1, 8, 15, and 22; daunorubicin 45 mg·m−2·d−1, days 1–3 and 15–17; cyclophosphamide 750 mg·m−2·d−1, days 1 and 15; and prednisone 1 mg·kg−1·d−1, days 1–28) [Citation10] and one kind of TKIs (imatinib 400–600 mg·d−1 or dasatinib 100–120 mg·d−1 or nilotinib 600–800 mg·d−1) was administered once the Philadelphia chromosome or the fusion gene of BCR/ABL was demonstrated. The third-generation TKIs would be given to patients who harbored BCR-ABL T315I mutation. Patients, who reached CR, were treated with consolidation chemotherapy, which contains several regimens, such as high-dose methotrexate (2 g·m−2·d−1, day1), CAM (cyclophosphamide 750 mg·m−2·d−1, days 1 and 15; arabinoside cytarabine 200 mg·m−2·d−1, days 1–3 and days 8–10; 6-mercaptopurine 60 mg·m−2·d−1, generally 100 mg/d, days 1–7), DOAME (dexamethasone 0.15 mg·kg−1·d−1, days 1–5; vincristine 1.4 mg·m−2·d−1, maximum 2 mg/d, day 1; arabinoside cytarabine 2 g·m−2·d−1, days 1–3; mitoxantrone 8 mg·m−2·d−1, days 2 and 3; etoposide 0.1 g/d, days 3–5), etc. Lumbar puncture and intrathecal chemotherapy were performed once completed remission was achieved. TKIs were administered till 2 weeks before transplantation.
Graft preparation
Donor peripheral blood stem cells (PBSC) were collected following G-CSF mobilization (5–10 μg·kg−1·d−1 for 5–6d) for allo-SCT recipients. Auto-SCT recipients received auto-PBSCs harvested after chemotherapy and recombinant G-CSF-induced mobilization. DOAME regimen (as mentioned above) was used in mobilization. Four patients and 2 donors failed PBSC mobilization (CD34+ cells < 1 × 106/kg within 2 collection days) and bone marrow stem cells were collected as complement.
Transplant protocols
The myeloablative conditioning based on total body irradiation (TBI) was administered to most patients (n = 100): TBI (7Gy day −7, or 3.3Gy for days −9, −8, −7), cyclophosphamide (40 mg·kg−1·d−1, days −6, −5), fludarabine (30 mg·m−2·d−1, days −4,−3, −2) and cytarabine (2 g·m−2·d−1, days −4, −3, −2). Nineteen patients were administered myeloablative conditioning based on busulfan of which TBI was replaced with busulfan (3.2 mg·kg−1·d−1, days −9, −8, −7).
Median mononuclear cells and CD34+ cells infused were 5.18 × 108/kg (range 1.32–22.00 × 108/kg) and 2.45 × 106/kg (range 1.44–6.09 × 106/kg) in the auto-SCT group, 8.00 × 108/kg (range 2.00–23.76 × 108/kg) and 2.66 × 106/kg (range 1.30–10.99 × 106/kg) in the MSD-SCT group, 10.00 × 108 /kg (range 7.30–21.5 × 108 /kg), and 2.8 × 106/kg (range 1.99–6.14 × 106/kg) in the haplo-SCT group.
GVHD prophylaxis for MSD-SCT recipients comprised of cyclosporine A (1 mg·kg−1·d−1) or tacrolimus (0.03 mg·kg−1·d−1) starting at day −1 and methotrexate (MTX, 15 mg·m−2 on day +1; and 10 mg·m−2 on days + 3, + 6). For haplo-SCT recipients, cyclosporine A or tacrolimus was started at day −5 and one dose of methotrexate was additionally administered at day +11. Meanwhile, mycophenolate mofetil (MMF, 15 mg·kg−1·d−1,since day −9) and ATG (rabbit, 2.5 mg·kg−1·d−1,days −4,−3,−2,−1) were also applied for prophylaxis. MMF was discontinued at day 60+ in the absence of GVHD. GVHD was diagnosed according to the Seattle criteria [Citation11,Citation12] and glucocorticoid was used as first-line treatment.
Maintenance therapy after transplantation for auto-SCT recipients
When WBC reached 3 × 109/L and platelets reached 50 × 109/L after auto-SCT, patients began to be given the maintenance therapy which may be continued for 1–1.5 years. The maintenance therapy generally based on TKIs combined with VDP/VIP regimen (vincristine 1.4 mg·m−2, d1, d8; daunorubicin 20–30 mg·m−2 d1,d8 or idamycin 6 mg·m−2 d1,d8; prednisone 1 mg·kg−1, d1–14) or MM regimen (MTX 15–20 mg·m−2, d1,d8; 6-mercaptopurine 50–75 mg·m−2, d1–14) alternatively. No maintenance therapy was given to the allo-SCT recipients until MRD turned positive or leukemia relapsed.
Definitions
CMR was defined as BCR-ABL in bone marrow specimens decreased below the detection limit of RT–PCR (BCR-ABL < 0.01% was defined negative, the sensitivity of testing MRD was 0.0032%), while s3CMR was defined as the achievement of CMR within 3 months of treatment and the CMR must be sustained up to HSCT. If CMR was achieved at 3 months but lost at any time before HSCT, it was also defined as non-s3CMR. Neutrophil engraftment was defined as the neutrophil count >0.5 × 109/L for 3 consecutive days and the first day was the date of neutrophil engraftment. Platelet engraftment was defined as platelet count >20 × 109 /L without platelet transfusion for 7 consecutive days and the first day was the date of platelet engraftment. Overall survival was defined as the duration from stem cell transplantation to death of any cause or last follow-up. Leukemia-free survival was calculated from the time of transplantation until relapse, death or last follow-up. Non-relapse mortality was defined as death without leukemic relapse. Relapse was defined as recurrence of lymphoblasts in the bone marrow (>5%), any lymphoblasts in the peripheral blood (hematological relapse) or molecular relapse.
Statistical analysis
For comparison of patients’ characteristics, Chi-square test (categorical variables) and the Kruskal–Wallis test (continual variables) were used. The probabilities of OS and LFS were estimated by the Kaplan–Meier method and the difference between subgroups was calculated with log-rank test. Relapse and NRM rates were estimated by the cumulative incidence analysis and compared by Gray’s test with relapse and NRM being competing events. Cox proportional regression model and competing risk model were used for multivariate analysis of risk factors for survival. Variables with p < 0.15 were included in multivariate analyses. All tests were two-sided, and p < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 22.0 for Windows (IBM, Armonk, NY) and R 3.5.1 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient characteristics
A total of 119 Ph+ ALL patients were included in this study, including 42 recipients of auto-SCT, 60 recipients of MSD-SCT and 17 recipients of haplo-SCT. Compared to MSD-SCT and haplo-SCT group, higher proportion of recipients in the auto-SCT group achieved CMR and s3CMR (CMR: 70.0% vs 64.7% vs 95.2%, p = 0.001; s3CMR: 43.3% vs 41.2% vs 76.2%, p = 0.002). The median time from diagnosis to transplantation was 9 (5–20) months in the auto-SCT group, 7 (3–35) months in the MSD-SCT group and 7 (4–11) months in the haplo-SCT group, respectively (P<0.001). The median chemotherapy cycles were 5 in auto-SCT, 4 in the MSD-SCT group and 5 cycles in the haplo-SCT group (p < 0.001). These three groups were comparable in terms of gender, age, disease status, white blood cell (WBC) count, platelet (PLT) count, karyotype and gene mutation at diagnosis. Most of the patients (n = 103) took only one kind of TKI, while the remaining 16 patients took an alternative TKI of a second or a third-generation TKI due to disease development or intolerance of the initial TKIs. Four patients took ponatinib as they harbored T315I mutation. Among the recipients who only administered one kind of TKI before transplantation, 60 patients (81.1%) achieved CMR in the imatinib group as compared to 25 patients (86.2%) in dasatinib/nilotinib group (p = 0.743), while the percent of achieving s3CMR was 59.5% vs 65.5% in these two groups, respectively (p = 0.570). The clinical characteristics of the patients are summarized in .
Table 1. Patient characteristics.
Hematopoietic reconstitution and GVHD
One patient failed neutrophil reconstitution and 3 patients failed PLT recovery. The median time to neutrophil engraftment was 14(10–29) days and the median time to PLT recovery was 19(10–87) days. In allo-SCT group, the incidence of II-IV and III-IV acute GVHD was 28.3% and 8.3% in MSD-SCT, 47.1% and 11.8% in the haplo-SCT group, respectively. The incidence of limited and extensive chronic GVHD was 25% versus 23.5% and 13.3% versus 0% in MSD-SCT and haplo-SCT group. For the auto-SCT group, the maintenance therapy generally began after hematopoietic reconstitution based on TKIs combined with VDP/VIP regimen and MM regimen alternatively. The patients usually applied 8–10 maintenance treatments a year as it took almost 2.5–3 months to achieve one cycle of VDP/VIP and MM regimen, including 2 weeks of chemotherapy and 2–4 weeks of blood recovery for each regimen.
Long-term survival
The median follow-up for all the participants was 27 (1–122) months after transplantation. The estimated OS at 3 years was not significantly different among 3 groups, 65.9 ± 8.8% after auto-SCT, 74.3 ± 6.0% after MSD-SCT and 81.9 ± 9.5% after haplo-SCT (p = 0.960). The estimated LFS at 3 years did not differ significantly either in three groups, 55.9 ± 8.6% after auto-SCT, 55.7 ± 6.7% after MSD-SCT and 67.2 ± 12.3% after haplo-SCT (p = 0.917) (; ). Multivariate analysis found that the risk of overall survival would decrease with lower WBC count (WBC < 100 × 109/L) and s3CMR state, while LFS was only associated with s3CMR ( and ).
Figure 1. Results of stem cell transplantation for patients with Philadelphia-positive ALL according to donor type. (A) overall survival, (B) leukemia-free survival, (C) cumulative incidence of relapse and (D) non-relapse mortality.
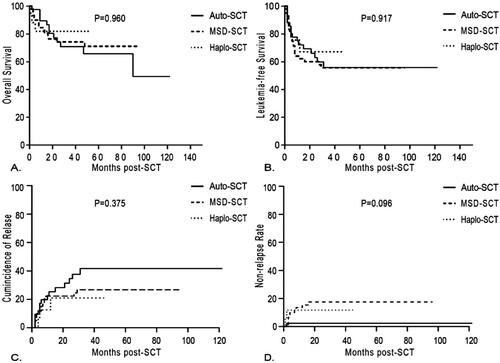
Table 2. Univariate analysis of factors related to OS, LFS, RI and NRM.
Table 3. Multivariate analysis of factors related to OS, LFS and CIR.
Table 4. Multivariate analysis of factors related to OS, LFS and CIR including the type of SCT.
Relapse and NRM
At the end of follow-up, 35.7% patients in the auto-SCT group underwent relapse at a median time of 6 months (range, 2–31 months) after transplantation, 25.0% patients in the MSD-SCT group relapsed at a median time of 6 months (range, 2–29 months) and 17.6% patients in the haplo-SCT group relapsed at a median time of 5 months (range, 4–12 months). The CIR rate at 3 years was 41.7 ± 8.7% after auto-SCT, 26.8 ± 6.1% after MSD-SCT and 21.0 ± 11.5% after haplo-SCT. Recipients of auto-SCT demonstrated higher CIR than those of allo-SCT, but the difference was not significant (p = 0.375). Recipients of MSD-SCT and haplo-SCT demonstrated higher NRM than those of auto-SCT, but the difference was not significant either (17.5% vs 11.8% vs 2.38% at 3 years, p = 0.096) (). The causes of NRM in the MSD-SCT and haplo-SCT group were infection (n = 9) and GVHD (n = 3), while only one patient died of infection in the auto-SCT group. Multivariate analysis found that patients who achieved s3CMR and underwent allo-SCT were prone to get lower relapse rate ( and ).
Patients who could benefit from auto-SCT
Multivariate analysis found that s3CMR was the important factor associated with the prognosis ( and ). The estimated 3-year CIR rate was lower in patients who achieved s3CMR (n = 65) than the non-s3CMR patients (n = 54) (17.6 ± 5.2% vs 49.8 ± 8.0; p = 0.001). The patients with s3CMR also had significantly higher LFS and higher OS as compared with patients without s3CMR at 3 years after SCT (70.8 ± 6.1% vs 38.4 ± 7.7%, p = 0.001; 78.6 ± 5.6% vs 67.2 ± 7.1%, p = 0.047) ().
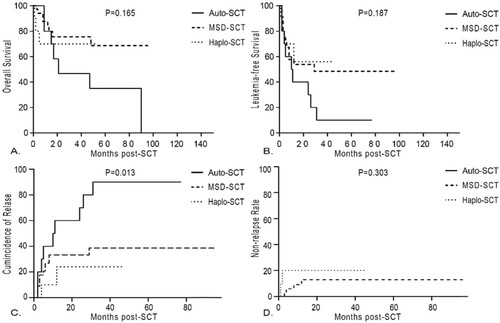
For the 65 patients who achieved s3CMR, there was no significant difference in OS (84.5 ± 7.2% vs 72.5 ± 8.9% vs 100%, p = 0.374), LFS (75.2 ± 8.3% vs 64.5 ± 9.5 vs 83.3 ± 15.2%, p = 0.668), CIR (17.2 ± 7.2% vs 8.1 ± 5.6% vs 16.7 ± 16.7%, p = 0.583) and NRM (3.1 ± 3.1% vs 23.4 ± 8.6% vs 0%, p = 0.055) among auto-SCT group (n = 32), MSD-SCT group (n = 26) and haplo-SCT group (n = 7) (). It was worth mentioning that there was a trend of lower NRM in the auto-SCT group although the difference was not significant. But in patients who did not achieve s3CMR, auto-SCT recipients tended to have higher RR (60.0 ± 16.9% vs 33.2 ± 8.4% vs 24.0 ± 16.6%, p = 0.013), lower LFS (30.0 ± 14.5% vs 53.8 ± 8.9% vs 56.0 ± 17.1%, p = 0.187) and lower OS (46.7 ± 16.6% vs 72.5 ± 8.9% vs 70.0 ± 14.5%, p = 0.165) than the MSD-SCT and haplo-SCT group (). Moreover, in 7 patients who achieved s3CMR in the haplo-SCT group, patients all survived and in those who did not achieve s3CMR, the causes of death were aGVHD (1/10), infection (1/10) and relapse (1/10). In the MSD-SCT group, patients who achieved s3CMR died of infection (5/26), relapse (1/26) and aGVHD (1/26) and in those who did not achieve s3CMR, patients died of relapse (4/34), infection (3/34) and aGVHD (1/34). While in the auto-SCT group, patients who did not achieve s3CMR all died of relapse. For those who achieved s3CMR, only 1 patient died of infection.
Figure 2. Results of Philadelphia-positive ALL patients with s3CMR according to donor type. (A) overall survival, (B) leukemia-free survival, (C) cumulative incidence of relapse and (D) non-relapse mortality.
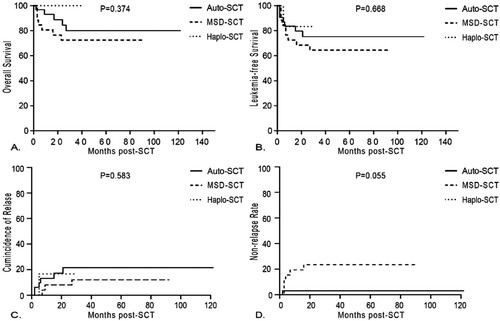
Figure 3. Results of Philadelphia-positive ALL patients with non-s3CMR according to donor type. (A) overall survival, (B) leukemia-free survival, (C) cumulative incidence of relapse and (D) non-relapse mortality.
The data suggested that patients with s3CMR could benefit from auto-SCT, for non-s3CMR patients, allogeneic transplantation may be more effective from lower relapse. It should be noted that all the auto-SCT patients in this study had received 1–1.5y maintenance treatment after hematopoietic reconstruction, which may be one of the factors to reduce relapse.
Mutational spectrum in Ph+ ALL
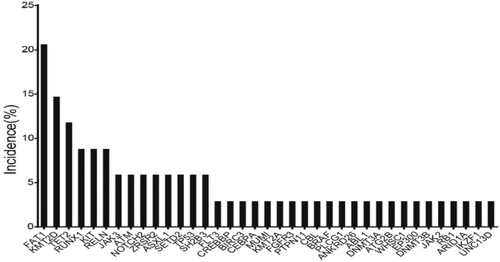
In recent years, with the application of next-generation sequencing (NGS) technology, genomics has been extensively developed in ALL patients. In this study, 34 patients were tested for second-generation sequencing, of which 6 (17.6%) patients were negative and the other 28 patients (82.4%) had at least one genetic mutation with a median number of 2 (0–5) mutation. Eleven patients (32.4%) harbored at least 3 gene mutations. The six most frequently mutated genes were FAT1, KMT2D, TET2, RUNX1, KIT and RELN ().
The main signaling pathways involved in this NGS gene panel were transcription factor/regulator, Ras/protein phosphatase/MARK signaling pathway, JAK-STAT pathway, splicing and mRNA processing regulation, epigenetic modulators and so on. Frequencies of different signaling pathways involved are listed in .
Table 5. Signaling pathways in Ph+ ALL.
Among 34 patients, the median overall survival (OS) was 14.5 (4–46) months, median leukemia-free survival was 11 (2–46) months, median relapse time was 4 (2–8) months. The 3-year OS and RFS rates were 83.3% and 77.6%, respectively. The 3-year CIR and NRM rates were 16.4% and 6.0%, respectively. Neither mutated genes nor signaling pathways had an effect on OS, LFS, CIR and NRM. However, patients with JAK-STAT pathway might be more difficult to achieve CMR before HSCT (p = 0.072).
Discussion
The past decade has witnessed a significant leap of prognosis in Ph+ ALL thanks to the incorporation of TKIs into chemotherapy [Citation13–15]. The combination of imatinib with chemotherapy has improved the rate and depth of remission and extended the duration of remission as well [Citation16–18]. CMR rates were reported to be 78% with ponatinib, 28%−50% with imatinib, and 45%–65% with dasatinib [Citation19]. UKALL/ECOG 2993 study confirmed that allo-HSCT still remains the standard consolidation therapy to date [Citation1]. While in other studies, the dominance of allo-HSCT has been challenged only under certain circumstances. In the GRAALL study, allo-HSCT was associated with a significant benefit in relapse-free survival (RFS) (HR, 0.69; p = 0.036) and OS (HR, 0.64; p = 0.02) while patients with CMR had comparable RFS regardless of whether or not they underwent allo-HSCT [Citation20]. Similarly, in the nilotinib study, patients who underwent allo-HSCT had higher 2-year RFS than those receiving only chemotherapy + TKI (78% vs 49%, p = 0.045), but there was no difference in the 2-year estimated OS (80% vs 72%, p = 0.227). Among the 76 patients with CMR, the RFS was similar between allo-HSCT and non-allo-HSCT patients (53% vs 65%, p = 0.783) [Citation21]. Taken together, these data indicate the feasibility of chemotherapy + TKI maintaining treatment in patients with CMR in Ph+ ALL. Due to higher NRM following HLA mismatched SCT and lower RFS in chemotherapy + TKI treatment, alternative strategies are warranted and auto-SCT is one of the options, especially for patients with lower risk of leukemic relapse [Citation22]. Many studies have reported promising results of auto-SCT, compared with allo-SCT [Citation8,Citation9,Citation23]. Compared with those of allo-SCT, the benefits of auto-SCT lie in lower transplant-related mortality and complications, no GVHD and better quality of life; but the lack of graft-versus-leukemia effect and the potential possibility of contamination of leukemic cells in the graft are major defects [Citation24]. Therefore, auto-SCT might be just effective for some appropriate patients compared to allo-SCT.
In this study, firstly we compared the overall results of patients with Ph+ ALL who received auto-SCT, MSD-SCT and haplo-SCT as consolidation therapy at our center. The probabilities of LFS and OS did not differ significantly for those three study groups. However, higher relapse rate of the auto-SCT group counterbalanced the benefit in terms of NRM compared to the allo-SCT group. In our study, patients with s3CMR were defined as those who achieved CMR within 3 months of treatment and the CMR must be sustained prior to SCT. Patients, who didn’t achieve CMR within 3 months or those who got CMR at 3 months but did not continue to transplantation, were defined as non-s3CMR. The s3CMR was the only factor associated with OS, LFS and CIR in multivariate analysis. Patients with s3CMR showed higher LFS, higher OS and lower RR as compared to the non-s3CMR patients after transplantation.
The importance of MRD in Ph+ ALL before and after allo-SCT has already been confirmed and may be important for auto-SCT recipients as well [Citation25,Citation26]. A recent study found that MRD status at 3 months had better discrimination for long-term survival and relapse-free survival than MRD status at CR following SCT which was consistent with our results. In Short’s study, patients who achieved CMR by 3 months had superior survival as compared to those with lesser molecular responses, regardless of consolidation with SCT [Citation27]. So, whether a patient could achieve s3CMR or not was closely related to his prognosis. In our study, there were 14 patients who achieved CMR at 3 months but failed before transplantation, two of them underwent autologous SCT and relapsed. Other 12 patients received allo-SCT and 4 relapsed, 1 died of infection.
Secondly, we divided the patients into different groups according to the s3CMR. For all patients with s3CMR, the probabilities of LFS, OS and CIR at 3 years did not differ significantly between patients after auto-SCT and patients after allo-SCT; however, in the non-s3CMR patients, auto-SCT recipients tended to have higher RR than the allo-SCT recipients. Therefore, monitoring MRD, especially s3CMR, had great impact on the prognosis of the disease and the assessment of treatment response. Patients with s3CMR could be administered auto-SCT in the absence of matched sibling donors, but for those who didn’t achieve s3CMR, it would be better to undergo allo-SCT. What needs to be emphasized is that all the patients after auto-SCT would receive maintenance therapy as soon as WBC and PLT reached the certain count and the maintain therapy would be continued for 1–1.5 years which might be one of effective methods to reduce the relapse after auto-SCT in our study.
Meanwhile, we assessed the impact of TKIs on the CMR rate of patients with Ph+ ALL briefly. Compared to chemotherapy with imatinib, patients received chemotherapy with dasatinib/nilotinib showed a trend of achieving higher rates of MRD negativity in 3 months and before transplant. So combinations of second-generation TKIs and chemotherapy might debulk the leukemic clones more rapidly and more deeply just like the efficiency of second-generation TKIs in chronic myeloid leukemia [Citation28,Citation29]. The more rapidly and more deeply of CMR the patients achieved, the more choices of treatment including auto-SCT and the more encouraging outcomes the patients would get.
We also collected genetic mutations of Ph+ ALL patients through next-generation sequencing technology to identify potential molecular targets. In our study, the most obvious mutations in over 20% of the patients were FAT1 followed by KMT2D, TET2, RUNX1, KIT and RELN, while in another study, CRLF2, SF1, EP300 and CREBBP genes were also mutated at higher incidences [Citation30]. Our results unravel a yet unreported high frequency (over 40%) of alterations in epigenetic regulators in Ph+ ALL. Mutations in epigenetic signaling pathways have been associated with lower OS and LFS in Ph+ ALL without stem cell transplantation [Citation31]. However, neither mutated genes nor signaling pathways had an effect on OS, LFS, CIR, NRM and selection of HSCT treatment in our study. Only patients with mutation in JAK-STAT pathway might be more difficult to achieve CMR before HSCT (p = 0.072). Mutational spectrum for a larger number of Ph+ ALL patients and further research studies on their prognosis significance in HSCT are needed.
Our study had some limitations given its retrospective nature and low case numbers, especial there was no analysis of outcomes from unrelated donor transplantation due to the limited patients. Further investigations are also needed for the choice between auto-SCT and TKIs-containing chemotherapy as consolidation for patients achieving s3CMR.
In summary, the s3CMR was emphasized for the speed and sustainability of complete molecular remission in our study which is different from just CMR at 3 months with other research studies. In addition, our data suggested that in the era of TKI, auto-SCT followed by maintenance therapy for 1–1.5 years could be an alternative consolidation option for Ph+ ALL patients especially for those s3CMR was kept up to transplant. But for non-s3CMR patients, allogeneic transplantation may be more effective and beneficial for reducing relapse.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Saini L, Brandwein J. New treatment strategies for Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2017;12(2):136–142.
- Tekgunduz E, Goker H, Kaynar L, et al. Adult Philadelphia chromosome-positive acute lymphoblastic leukemia in daily practice: a multicenter experience. Clin Lymphoma Myeloma Leuk. 2016;16(5):269–274.
- Short NJ, Kantarjian H, Jabbour E, et al. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2017;30(3):193–200.
- Ribera JM. Optimal approach to treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: how to best use all the available tools. Leuk Lymphoma. 2013;54(1):21–27.
- Ravandi F. Managing Philadelphia chromosome-positive acute lymphoblastic leukemia: role of tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk. 2011;11(2):198–203.
- Brissot E, Labopin M, Beckers MM, et al. Tyrosine kinase inhibitors improve long-term outcome of allogeneic hematopoietic stem cell transplantation for adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):392–399.
- Gao L, Zhang C, Gao L, et al. Favorable outcome of haploidentical hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia: a multicenter study in southwest China. J Hematol Oncol. 2015;8:90.
- Giebel S, Labopin M, Gorin NC, et al. Improving results of autologous stem cell transplantation for Philadelphia-positive acute lymphoblastic leukaemia in the era of tyrosine kinase inhibitors: a report from the Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Eur J Cancer. 2014;50(2):411–417.
- Giebel S, Labopin M, Potter M, et al. Comparable results of autologous and allogeneic haematopoietic stem cell transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia in first complete molecular remission: An analysis by the Acute Leukemia Working Party of the EBMT. Eur J Cancer. 2018;96:73–81.
- Ding Z, Han MZ, Chen SL, et al. Outcomes of adults with acute lymphoblastic leukemia after autologous hematopoietic stem cell transplantation and the significance of Pretransplantation minimal residual disease:analysis from a single center of China. Chin Med J (Engl). 2015;128(15):2065–2071.
- Joachim H, Deeg MD, Rainer Storb MD. Graft-versus-host disease: pathophysiological and clinical aspects. Ann Rev Med. 1984;34:11–24.
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233.
- Fielding AK. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2010;116(18):3409–3417.
- Xavier Thomas MH. Diagnostic and treatment of adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol Oncol. 2016;5(2):77–90.
- Malagola M, Papayannidis C, Baccarani M. Tyrosine kinase inhibitors in Ph+ acute lymphoblastic leukaemia: facts and perspectives. Ann Hematol. 2016;95(5):681–693.
- Ronson A, Tvito A, Rowe JM. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia. Curr Treat Options Oncol. 2017;18(3):20.
- Hatta Y, Mizuta S, Matsuo K, et al. Final analysis of the JALSG Ph + ALL202 study: tyrosine kinase inhibitor-combined chemotherapy for Ph + ALL. Ann Hematol. 2018;97(9):1535–1545.
- Fujisawa S, Mizuta S, Akiyama H, et al. Phase II study of imatinib-based chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Am J Hematol. 2017;92(4):367–374.
- Fakih R E, Jabbour E, Ravandi F, et al. Current paradigms in the management of Philadelphia chromosome positive acute lymphoblastic leukemia in adults. Am J Hematol. 2018;93(2):286–295.
- Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–3719.
- Kim DY JY, Lim SN, Kim SD, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746–756.
- Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1):150–155.
- Wetzler M, Watson D, Stock W, et al. Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB study 10001 (alliance). Haematologica. 2014;99(1):111–115.
- Chen J, Yang L, Fan Y, et al. Comparison of autologous stem cell transplantation versus haploidentical donor stem cell transplantation for favorable- and intermediate-risk acute myeloid leukemia patients in first complete remission. Biol Blood Marrow Transplant. 2018;24(4):779–788.
- Leonard JT, Stock W. The Persistence of minimal residual disease in Philadelphia chromosome-positive acute lymphoblastic leukemia: we know it’s bad, now what? Biol Blood Marrow Transplant. 2016;22(11):1913–1914.
- Nishiwaki S, Imai K, Mizuta S, et al. Impact of MRD and TKI on allogeneic hematopoietic cell transplantation for Ph+ ALL: a study from the adult ALL WG of the JSHCT. Bone Marrow Transplant. 2016;51(1):43–50.
- Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–507.
- Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333–2340.
- Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054.
- Feng J, Li Y, Jia Y, et al. Spectrum of somatic mutations detected by targeted next-generation sequencing and their prognostic significance in adult patients with acute lymphoblastic leukemia. J Hematol Oncol. 2017;10(1):61.
- Feng J, Gong XY, Jia YJ, et al. Spectrum of somatic mutations and their prognostic significance in adult patients with B cell acute lymphoblastic leukemia. Zhonghua xue ye xue za zhi = Zhonghua Xueyexue Zazhi. 2018;39(2):98–104.