ABSTRACT
Objective
This study aimed to investigate the correlations of long non-coding RNA ANRIL (lncRNA ANRIL), microRNA (miR)-34a, miR-125a and miR-186 with disease risk, clinical features and prognosis of multiple myeloma (MM).
Method
Totally, 87 MM patients and 30 controls were recruited. LncRNA ANRIL and its target miRNAs (miR-34a, miR-125a and miR-186) in bone marrow derived plasma cells were detected by RT-qPCR. Treatment response was assessed and survivals were calculated in MM patients.
Results
LncRNA ANRIL expression was increased, while miR-34a, miR-125a and miR-186 expressions were reduced in MM patients compared with controls. Meanwhile, lncRNA ANRIL negatively correlated with miR-34a and miR-125a but not miR-186 in MM patients, while did not correlate with miR-34a, miR-125a or miR-186 in controls. In MM patients, lncRNA ANRIL high expression associated with higher beta-2-microglobulin (β2-MG) and more advanced international staging system (ISS) stage; miR-125a high expression associated with lower β2-MG, less advanced ISS stage and less t (14; 16) abnormality; miR186 high expression associated with increased albumin; while miR-34a did not associate with any clinical features. Furthermore, lncRNA ANRIL high expression associated with decreased complete response (CR), while miR-34a high and miR-125a high expression associated with increased CR and objective response rate. Additionally, lncRNA ANRIL high expression associated with shorter progression-free survival (PFS), while miR-34a high expression associated with prolonged overall survival (OS), and miR-125a high expression associated with longer PFS and OS.
Conclusion
LncRNA ANRIL and its target miRNAs might serve as biomarkers for assisting with personalized treatment and prognosis improvement of MM.
Introduction
Multiple myeloma (MM), a hematological malignancy of B lymphocytes, is marked by the proliferation of clonal plasma cells in the bone marrow [Citation1,Citation2]. It is the second most common hematological malignant abnormality, which is clinically manifested as bone pain, hypercalcemia, anemia and renal insufficiency [Citation1,Citation3]. In recent years, the advent of autologous stem cell transplantation, proteasome inhibitors and immunomodulatory drugs has progressively and greatly increased the survival of patients with MM [Citation3,Citation4]. Despite the achievement of improved prognosis, most of patients with MM will inevitably relapse or become refractory to current treatments due to the heterogeneity of disease, which remain as major obstacles for treating patients with MM [Citation5]. To address this, it is pressingly needed to explore the potential biomarkers for guiding personalized treatment strategies and improving prognosis in patients with MM.
Long non-coding RNA antisense non-coding RNA in the INK4 locus (LncRNA ANRIL) is a cancer-related long non-coding RNA that is transcribed from the short arm of chromosome 9 on p21.3, which is involved in the development and progression of hematological malignancies [Citation6–10]. As an example, in AML, lncRNA ANRIL facilitates the survival of malignant cells to accelerate disease progression [Citation8]. Another study illustrates that lncRNA ANRIL associates with relapse in MM patients after autologous stem cell transplant [Citation7]. Meanwhile, previous studies illuminate that lncRNA ANRIL enhances cell proliferation, migration and invasion to participate in the pathogenesis of cancers by interacting with microRNA (miR)-34a, miR-125a or miR-186; additionally, miR-34a, miR-125a and miR-186 are reported as tumor suppressors in the pathogenesis of MM [Citation9,Citation11–15]. Based on above-mentioned evidence, it was hypothesized that lncRNA ANRIL might be clinically implicated in MM via interacting with miR-34a, miR-125a and miR-186. However, relevant studies are lacking. Therefore, this present study was to investigate the correlations of lncRNA ANRIL, its target miRNAs (miR-34a, miR-125a and miR-186) with disease risk, clinical features and prognosis in MM patients, aiming to provide new biomarkers for helping with clinical management of these patients.
Methods
Participants
This study recruited 87 de novo symptomatic MM patients and 30 controls. The MM patients were consecutively enrolled from our hospital from January 2016 to October 2019, and the controls were screened from bone marrow donors in our hospital from January 2018 to December 2019. The inclusion criteria of MM patients were as follows: (1) newly diagnosed as de novo symptomatic MM based on International Myeloma Working Group (IMWG) criteria of multiple myeloma [Citation16]; (2) aged ≥18 years old. The exclusion criteria included that: (1) asymptomatic MM, relapsed MM or MM complicated by amyloidosis; (2) complicated with other hematologic malignancies or solid tumors; (3) infected with human immunodeficiency virus or other fatal virus; (4) history of chemotherapy, radiotherapy or stem cell transplantation; (5) pregnant or lactating women. The healthy conditions of controls were confirmed when they were at bone marrow donation examination, and the major screening criteria were that: (1) no history of hematologic malignancies or solid tumors; (2) aged ≥18 years old; (3) no blood-borne infectious diseases (e.g. hepatitis B, hepatitis C, acquired immune deficiency syndrome, syphilis); (4) no severe heart, liver and kidney function disorders; (5) no obvious abnormal in biochemical examination. This study was approved by the Ethics Committee of our hospital, and the participants signed written informed consents.
Data and sample collection
After signing the written informed consents, the clinical features of MM patients were collected, which included that: age, gender, immunoglobulin subtype (immunoglobulin (Ig) A, IgG and others), renal impairment, biochemical index (such as hemoglobin (Hb), calcium, serum creatinine (Scr), albumin (ALB), beta-2-microglobulin (β2-MG) and lactate dehydrogenase (LDH)), clinical stage (Durie-Salmon stage and international staging system (ISS) stage), and chromosomal abnormalities (such as t (4; 14), t (14; 16) and Del (17p)). The bone marrow samples of MM patients were collected before initial treatment, and the bone marrow samples of controls were acquired on the examination of the eligibility for bone marrow transplantation. After that, the bone marrow samples were processed with gradient density centrifugation to isolate bone marrow mononuclear cells (BMMCs). Then, the BMMCs were cultivated with CD138-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, North Rhine-Westphalia, Germany) to purify plasma cells, and the purity of isolated plasma cells was detected by flow cytometry analysis. The purity isolated plasma cells in patients with MM ranged from 90% to 99%, with the mean purity of approximately 97%. Meanwhile, the purity of isolated plasma cells in controls ranged from 75% to 95%, with the mean of approximately 80%. Finally, the plasma cells were stored in liquid nitrogen until further detection.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The relative expressions of lncRNA ANRIL, miR-34a, miR-125a and miR-186 in plasma cells were detected by RT-qPCR. Initially, total RNA was extracted from the plasma cells by TRIzol™ Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Then, the RNA was converted to the complementary DNA (cDNA) using iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, California, USA). After that, qPCR was performed using QuantiNova SYBR Green PCR Kit (Qiagen, Duesseldorf, Nordrhein-Westfalen, Germany). GAPDH was applied as the internal reference for lncRNA ANRIL, and U6 was applied as the internal reference for miR-34a, miR-125a and miR-186[Citation17–21]. Lastly, the relative expression of lncRNA ANRIL, miR-34a, miR-125a and miR-186 was calculated using by 2-△△Ct method. The primers applied were listed in supplementary table 1. Notably, the Ct value variant of GAPDH was 21% in patients with MM, and the Ct value variant of GAPDH was 19% in healthy donors; further comparison analysis of Ct value variant of GAPDH between patients with MM and healthy donors showed no difference. These findings indicated that GAPDH was relatively stable in both patients with MM and healthy donors.
Treatment and assessment
According to NCCN guideline of MM [Citation22], all patients received appropriate primary induction therapy regimen based on their clinical status. The primary induction therapy regimens were mainly bortezomib-based regimens as follows: (1) Bortezomib/dexamethasone (bortezomib at a dose of 1.3 mg/m2 on day 1, day 4, day 8, day 11; dexamethasone at a dose of 20 mg once a day on day 1, day 2, day 4, day 5, day 8, day 9, day 11, day 12; 21 days as a cycle); (2) Bortezomib/cyclophosphamide/dexamethasone (bortezomib at a dose of 1.3 mg/m2 on day 1, day 4, day 8, day 11; cyclophosphamide at a dose of 140 mg/m2 and dexamethasone at a dose of 20 mg/d on days 1–4; 21 days as a cycle); (3) Bortezomib or Dexamethasone/cyclophosphamide/etoposide/cisplatin (dexamethasone at a dose of 30 mg/day, cyclophosphamide at a dose of 400 mg/m2, etoposide at a dose of 40 mg/m2 and cisplatin at a dose of 10 mg/m2 on days 1–4; 28 days as a cycle); (4) Melphalan/prednisone/bortezomib (melphalan at a dose of 9 mg/m2 and prednisone at a dose of 60 mg/m2 on days 1–4; bortezomib at a dose of 1.3 mg/m2 on day 1, day 4, day 8, day 11, day 22, day 25, day 29, and day 32; 42 days as a cycle). After two cycles of primary induction therapy, clinical response of patient with MM was assessed referring to NCCN guideline of MM [Citation22], which included that: (1) complete response (CR): negative immunofixation in the serum and urine, as well as disappearance of any soft tissue plasmacytomas and ≤5% plasma cells in bone marrow; (2) very good partial response (VGPR): serum and urine M-protein detectable by immunofixation but not on electrophoresis or 90% or greater reduction in serum M-protein plus urine M-protein level <100 mg per 24 h; (3) partial response (PR): ≥50% reduction of serum M-protein and reduction in 24 h urinary M-protein by ≥90% or to< 200 mg per 24 h; (4) progressive disease (PD): increase of ≥25% from baseline (details were shown in NCCN guideline of MM); (5) stable disease (SD): not meeting criteria for CR, VGPR, PR or PD. The objective response rate (ORR) of patients was defined as the percentage of patients who achieved CR, VGPR or PR. After clinical response assessment, subsequent therapies were also conducted according to the NCCN guideline of MM [Citation22].
Follow-up
All patients were regularly followed up by telephone or clinical visit, with the last visit date at 2019/12/31. The median follow-up time of patients was 23.0 months, with a range of 2.0–42.0 months. According to the disease status or survival status of patients, progression-free survival (PFS) and overall survival (OS) were calculated. PFS was defined as the duration from initial treatment to disease progression or death. OS was defined as the duration from initial treatment to death. During follow-up, the patients who died in primary induction therapy or had no clinical response data due to early lost follow-up were excluded from the final analysis. Besides, for the patients who were not known to have progressed or died at the last follow-up date, they were censored on the date of last visit or on the date of last known to be alive.
Statistical analysis
Statistical analyses were performed with the use of SPSS 24.0 SPSS 24.0 (IBM, Chicago, IL, USA). Figures were made using GraphPad Prism 8.01 (GraphPad Software Inc., San Diego, CA, USA). Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) based on their normality. Categorical data were displayed as count (percentage). Comparisons of lncRNA ANRIL, miR-34a, miR-125a and miR-186 between controls and patients with MM were determined by Wilcoxon rank sum test. Correlation of lncRNA ANRIL with miR-34a, miR-125a or miR-186 was determined by Spearman’s rank correlation test. For further analysis, the expressions of lncRNA ANRIL, miR-34a, miR-125a and miR-186 in patients with MM were classified as high expression and low expression based on their median values, respectively. Correlation of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with clinical characteristics or CR/ORR was determined by Chi-square test or linear by linear association test. Kaplan–Meier curve was plotted to display PFS and OS, and comparisons of PFS and OS between two groups were determined by Log-rank test. Notably, the median value of lncRNA ANRIL (2.17) was used to calculate the association of lncRNA ANRIL with PFS and OS. Independent factors associated with PFS and OS were analyzed by backward stepwise multivariate Cox’s proportional hazard regression model, and the following factors were included in the multivariate Cox’s proportional hazard regression model: age, gender, immunoglobulin subtype, renal impairment, Hb, calcium, Scr, ALB, β2-MG, LDH, Durie-Salmon stage, ISS stage, t (4; 14), t (14; 16), Del (17p), lncRNA ANRIL, miR-34a, miR-125a and miR-186. P value <0.05 was considered as significant.
Results
Clinical characteristics of patients with MM
There were 35 (40.2%) females and 52 (59.8%) males, with a mean age of 55.0 ± 8.6 years in patients with MM (). As for immunoglobulin subtype, 47 (54.1%), 21 (24.1%) and 19 (21.8%) patients with MM had IgG, IgA and other immunoglobulin subtypes, respectively. Regarding clinical stage, 10 (11.5%) and 77 (88.5%) patients with MM were with Durie–Salmon stages II and III, respectively; 22 (25.3%), 19 (21.8%) and 46 (52.9%) patients with MM exhibited ISS stages I, II and III, respectively. In terms of chromosomal abnormalities, 12 (13.8%), 4 (4.6%) and 12 (13.8%) patients with MM presented with t (4; 14), t (14; 16) and Del (17p) chromosomal abnormalities, respectively. The information about renal impairment and biochemical index (including Hb, calcium, Scr, ALB, β2-MG and LDH) was shown in .
Table 1. MM patients’ characteristics.
LncRNA ANRIL, miR-34a, miR-125a and miR-186 expressions between patients with MM and controls
LncRNA ANRIL expression (P < 0.001) (A) was increased, while miR-34a (P < 0.001) (B), miR-125a (P < 0.001) (C) and miR-186 (P = 0.010) (D) expressions were decreased in patients with MM compared to controls.
Figure 1. Differences in lncRNA ANRIL, miR-34a, miR-125a and miR-186 expressions between patients with MM and controls. Comparisons of lncRNA ANRIL (A), miR-34a (B), miR-125a (C) and miR-186 (D) expressions between patients with MM and controls. lncRNA ANRIL, long non-coding RNA antisense non-coding RNA in the INK4 locus; miR, microRNA; MM, multiple myeloma.
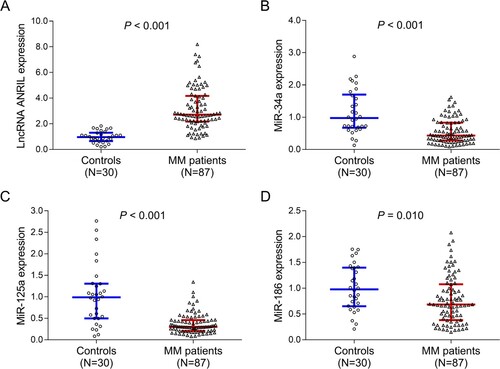
Correlation of lncRNA ANRIL with miR-34a, miR-125a and miR-186 in patients with MM and controls
In patients with MM, lncRNA ANRIL negatively correlated with miR-34a (P = 0.007, r = −0.287) (A) and miR-125a (P = 0.001, r = −0.361) (A), while it did not correlate with miR-186 (P = 0.293, r = −0.114) (C). In controls, lncRNA ANRIL did not correlate with miR-34a (P = 0.224, r = −0.229) (D), miR-125a (P = 0.498, r = −0.129) (E) or miR-186 (P = 0.651, r = −0.086) (F).
Figure 2. Correlation of lncRNA ANRIL with miR-34a, miR-125a and miR-186 in patients with MM and controls. Correlation of lncRNA ANRIL with miR-34a (A), miR-125a (B) and miR-186 (C) in patients with MM. Correlation of lncRNA ANRIL with miR-34a (D), miR-125a (E) and miR-186 (F) in controls. lncRNA ANRIL, long non-coding RNA antisense non-coding RNA in the INK4 locus; miR, microRNA; MM, multiple myeloma.
Associations of lncRNA ANRIL, mir-34a, mir-125a and mir-186 with clinical characteristics in patients with MM
In patients with MM, lncRNA ANRIL high associated with higher β2-MG level (P = 0.042), more advanced ISS stage (P = 0.046); miR-125a high associated with male (P = 0.040), lower β2-MG level (P = 0.007), less advanced ISS stage (P = 0.010) and less t (14; 16) abnormality (P = 0.038); miR-186 high associated with elevated ALB level (P = 0.035); while no association of miR-34a with any clinical characteristics was observed ().
Table 2. Correlations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with clinical characteristics.
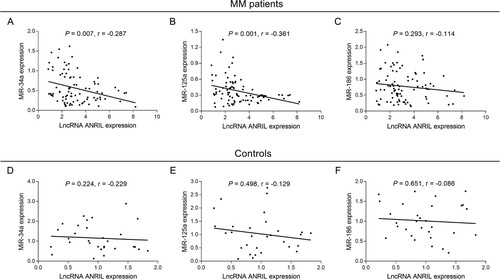
Associations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with treatment response in patients with MM
In patients with MM, lncRNA ANRIL high associated with reduced CR (P = 0.036) (A), while it did not associate with ORR (P = 0.064) (B); miR-34a high associated with both higher CR (P = 0.048) (C) and ORR (P = 0.003) (D); miR-125a high also associated with both elevated CR (P = 0.013) (E) and ORR (P = 0.047) (F); however, miR-186 did not associate with CR (P = 0.953) (G) or ORR (P = 0.132) (H).
Figure 3. Associations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with CR and ORR in patients with MM. Comparisons of CR and ORR between patients with lncRNA ANRIL low expression and patients with lncRNA ANRIL high expression (A, B), between patients with miR-34a low expression and patients with miR-34a high expression (C, D), between patients with miR-125a low expression and patients with miR-125a high expression (E, F), and between patients with miR-186 low expression and patients with miR-186 high expression (G, H). lncRNA ANRIL, long non-coding RNA antisense non-coding RNA in the INK4 locus; miR, microRNA; CR, complete response; ORR, objective response rate; MM, multiple myeloma.
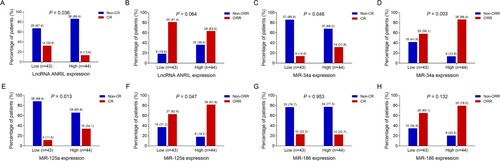
Associations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with survival profiles in patients with MM
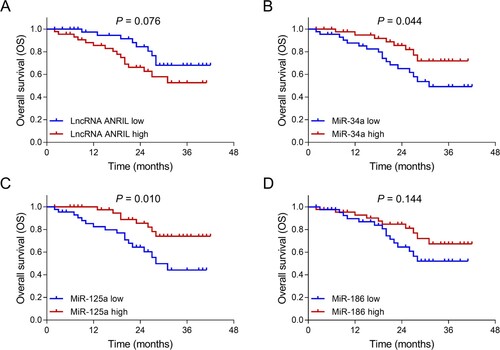
In patients with MM, lncRNA ANRIL high (P = 0.031) (A) associated with shorter PFS; miR-125a high (P = 0.011) (C) associated with prolonged PFS; while no association of miR-34a (P = 0.095) (B) or miR-186 (P = 0.483) (D) with PFS was observed. Meanwhile, miR-34a high (P = 0.044) (B) and miR-125a high (P = 0.010) (C) associated with longer OS, while there was no association of lncRNA ANRIL (P = 0.076) (A) or miR-186 (P = 0.144) (D) with OS ().
Figure 4. Associations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with PFS in patients with MM. Comparisons of PFS between patients with lncRNA ANRIL low expression and patients with lncRNA ANRIL high expression (A), between patients with miR-34a low expression and patients with miR-34a high expression (B), between patients with miR-125a low expression and patients with miR-125a high expression (C), between patients with miR-186 low expression and patients with miR-186 high expression (D). lncRNA ANRIL, long non-coding RNA antisense non-coding RNA in the INK4 locus; miR, microRNA; PFS, progression-free survival; MM, multiple myeloma.
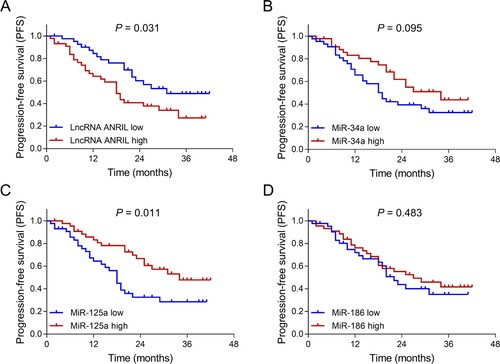
Figure 5. Associations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with OS in patients with MM. Comparisons of OS between patients with lncRNA ANRIL low expression and patients with lncRNA ANRIL high expression (A), between patients with miR-34a low expression and patients with miR-34a high expression (B), between patients with miR-125a low expression and patients with miR-125a high expression (C), between patients with miR-186 low expression and patients with miR-186 high expression (D). lncRNA ANRIL, long non-coding RNA antisense non-coding RNA in the INK4 locus; miR, microRNA; OS, overall survival; MM, multiple myeloma.
Associations of lncRNA ANRIL and its target miRNAs with survival profiles in patients with MM
In patients with MM, lncRNA ANRIL high&miR-125a low, lncRNA ANRIL high&miR-34a low&miR-125a low, lncRNA ANRIL high&miR-125a low&miR-186 low, lncRNA ANRIL high&miR-34a low&miR-186 low and lncRNA ANRIL high&miR-34a low&miR-125a low&miR-186 low were associated shorter PFS, while no association of lncRNA ANRIL high&miR-34a low or lncRNA ANRIL high&miR-186 low was PFS was observed (see Supplementary figure 1A–G). Meanwhile, lncRNA ANRIL high&miR-34a low, lncRNA ANRIL high&miR-125a low, lncRNA ANRIL high&miR-186 low, lncRNA ANRIL high&miR-34a low&miR-125a low, lncRNA ANRIL high&miR-125a low&miR-186 low, lncRNA ANRIL high&miR-34a low&miR-186 low and lncRNA ANRIL high&miR-34a low&miR-125a low&miR-186 low were associated with shorter OS, while there was no association of lncRNA ANRIL high&miR-186 low with OS (see Supplementary figure 2A–G). Collectively, these findings showed that lncRNA ANRIL combined single target miRNA, lncRNA ANRIL combined two target miRNAs and lncRNA ANRIL combined three target miRNAs were associated with shorter PFS and OS in patients with MM; meanwhile, the more target miRNAs that lncRNA ANRIL combined, the better it would predict the prognosis in patients with MM.
Independent factors for predicting survival profiles in patients with MM
Multivariate Cox’s regression analyses revealed that lncRNA ANRIL high (although it was available after backward stepwise multivariate Cox’s regression screening, no statistical significance was shown; P = 0.061), miR-34a, miR-125a and miR-186 could not independently predict PFS (). Meanwhile, among lncRNA ANRIL and its target miRNAs (miR-34a, miR-125a and miR-186), only miR-34a high (P = 0.049) independently predicted prolonged OS (). The detailed information about other independent factors of PFS and OS was displayed in and , respectively.
Table 3. Factors independently associated with PFS.
Table 4. Factors independently associated with OS.
Discussion
Existing studies disclose that lncRNA ANRIL serves as an oncogene in the pathogenesis of hematological malignancies, including AML and MM [Citation7–9]. For instance, in AML, lncRNA ANRIL enhances the survival of malignant cells to accelerate disease progression through regulating the glucose metabolism pathway of AdipoR1/AMPK/SIRT1 [Citation8]. As for MM, lncRNA ANRIL is reported to associate with relapse and primary myelofibrosis in patients with MM [Citation7,Citation10]. Meanwhile, based on previous studies, lncRNA ANIRL negatively regulates miR-34a, miR-125a and miR-186; moreover, miR-34a, miR-125a and miR-186 are reported to suppress cell proliferation while facilitate cell apoptosis in MM [Citation9,Citation11–15]. In the light of aforementioned evidence, lncRNA ANIRL might have clinical value in MM management via the interactions with miR-34a, miR-125a and miR-186, however, no related researches have been reported yet.
In the present study, the lncRNA ANRIL, miR-34a, miR-125a and miR-186 expression were detected and compared between patients with MM and controls, which displayed that lncRNA ANRIL expression was highly expressed, while miR-34a, miR-125a and miR-186 expressions were lowly expressed in patients with MM compared with controls. The following are possible explanations: (a) lncRNA ANRIL probably facilitated cell proliferation but repressed cell apoptosis by modulating the glucose metabolism pathway of AdipoR1/AMPK/SIRT1, which induced the malignant growth of plasma cells in MM, thereby, lncRNA ANRIL expression was elevated in MM patients compared with controls [Citation8]; and (b) miR-34a, miR-125a and miR-186 suppressed cell proliferation, colony formation and facilitated cancer stem cell apoptosis by downregulating transforming growth interaction factor 2, ubiquitin-specific peptidase 5 and Jagged1 protein, which in turn inhibited the growth of cells and tumors in MM, thereby, miR-34a, miR-125a and miR-186 expressions were lower in patients with MM than controls [Citation13–15]. Besides, lncRNA ANRIL negatively correlated with miR-34a and miR-125a in patients with MM. These data could be explained by that: LncRNA ANRIL might inhibit cell proliferation and facilitate cell apoptosis during MM pathogenesis via reversely regulating tumor suppressors (including miRNAs miR-34a and miR-125a), hence lncRNA ANRIL negatively correlated with miR-34a and miR-125a in patients with MM [Citation9,Citation11,Citation13,Citation15].
Furthermore, the present study assessed the associations of lncRNA ANRIL, miR-34a, miR-125a and miR-186 with clinical features in patients with MM. The results exhibited that lncRNA ANRIL high associated with higher β2-MG level and more advanced ISS stage; miR-125a high associated with reduced β2-MG level and decreased t (14; 16) abnormality; miR-186 associated with increased ALB level; while miR-34a did not associate with any clinical features. The possible explanations were that: (a) lncRNA ANRIL might promote the cell proliferation, migration, invasion and inhibit the cell apoptosis in AML via sponging multiple miRNAs (including miR-125a and miR-186), which contributed to exacerbated clinical features and poor risk stratification in patients with MM [Citation8,Citation11,Citation12]; (b) miR-125a and miR-186 might suppress the cell proliferation, survival and migration in MM by downregulating the expression of MM-associated oncogenes (such as IRF-4, XBP-1 and c-MYC), which alleviated disease progression and well risk stratification in patients with MM [Citation13–15,Citation23]; and (c) as the correlation of lncRNA ANRIL with miR-34a was weak, miR-34a did not associate with any clinical features, further validated study was needed.
The prognostic values of lncRNA ANRIL, miR-34a, miR-125a and miR-186 remain unknown in patients with MM. In the present study, we discovered that lncRNA ANRIL high expression associated with attenuated treatment response and survival; miR-34a high and miR-125a high expressions associated with elevated treatment response and survival; while miR-186 expression did not associate with treatment response or survival in patients with MM. These results could be explained by that: (a) as observed in the present study, lncRNA ANRIL expression associated with poor risk stratification (reflected by higher β2-MG level and more advanced ISS stage), which was related to poor prognosis in patients with MM; while, miR-125a expression associated with well risk stratification (reflected by lower β2-MG level, t (14; 16) abnormality and increased ALB level), which were linked with improved prognosis in patients with MM; (b) LncRNA ANRIL probably enhanced cell proliferation, migration and invasion while attenuated cell apoptosis and drug sensitivity of cancer cells, which maintained the survival of malignant cells, accelerated the disease progression and induced drug resistance, thereby, contributing to the poor treatment response and survival in patients with MM [Citation8,Citation9,Citation11,Citation12]; (c) MiR-34a and miR-125a probably repressed the MM cell growth, clone formation and facilitated cell apoptosis via the activation of pro-apoptotic P53 and c-Myc, which alleviated the malignancy and attenuated drug resistance, thereby, devoting into better prognosis in patients with MM [Citation13,Citation14,Citation23]; and (d) MiR-186 might have a weak correlation with ALB, which failed to predict prognosis in patients with MM. Notably, by further multivariate Cox’s regression analysis, lncRNA ANRIL high expression could not independently predict poor PFS, while miR-34a expression was an independent predictive factor for prolonged OS in MM patients, which might be explained by that lncRNA ANRIL interacted with miR-34a to participate in the pathogenesis of MM, thus, miR-34a but not lncRNA ARNIL could independently predict survival in patients with MM [Citation9].
However, the present study was subjected to some limitations. First, only 87 de novo symptomatic MM patients and 30 controls were enrolled. While the sample sizes were relatively small, which might result in poor statistical power, thereby, further validating studies with more MM patients and controls were needed. Second, only the expressions of lncRNA ANRIL, miR-34a, miR-125a and miR-186 before treatment were assessed in patients with MM, further investigations involving the longitudinal change of lncRNA ANRIL, miR-34a, miR-125a and miR-186 after the induction therapy would be desirable. Lastly, asymptomatic MM and relapsed MM patients were excluded from the enrollment, thus, our findings might not be applicable to these patients.
To conclude, lncRNA ANRIL and its target miRNAs (miR-34a, miR-125a and miR-186) reflect risk stratification or/and prognosis in patients with MM, which might assist with the disease management and the prognosis improvement in these patients.
Author’s contribution
Yafei Yin analyzed the data and contributed to writing. Wenqun Yang helped in drafting the manuscript and shaping the manuscript. Lu Zhang drafted the manuscript and helped in shaping the manuscript. Kang Liu provided valuable suggestions and helped in reviewing the manuscript. Zimian Luo designed the study and reviewed the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208.
- Kunacheewa C, Orlowski RZ. New drugs in multiple myeloma. Annu Rev Med. 2019;70:521–547.
- Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046.
- Paiva B, van Dongen JJ, Orfao A. New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood. 2015;125(20):3059–3068.
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91(1):101–119.
- Aguilo F, Di Cecilia S, Walsh MJ. Long non-coding RNA ANRIL and polycomb in human cancers and cardiovascular disease. Curr Top Microbiol Immunol. 2016;394:29–39.
- Poi MJ, Li J, Sborov DW, et al. Polymorphism in ANRIL is associated with relapse in patients with multiple myeloma after autologous stem cell transplant. Mol Carcinog. 2017;56(7):1722–1732.
- Sun LY, Li XJ, Sun YM, et al. LncRNA ANRIL regulates AML development through modulating the glucose metabolism pathway of AdipoR1/AMPK/SIRT1. Mol Cancer. 2018;17(1):127.
- Wang CH, Li QY, Nie L, et al. LncRNA ANRIL promotes cell proliferation, migration and invasion during acute myeloid leukemia pathogenesis via negatively regulating miR-34a. Int J Biochem Cell Biol. 2020;119:105666.
- Pennucci V, Zini R, Norfo R, et al. Abnormal expression patterns of WT1-as, MEG3 and ANRIL long non-coding RNAs in CD34+ cells from patients with primary myelofibrosis and their clinical correlations. Leuk Lymphoma. 2015;56(2):492–496.
- Zhang LM, Ju HY, Wu YT, et al. Long non-coding RNA ANRIL promotes tumorgenesis through regulation of FGFR1 expression by sponging miR-125a-3p in head and neck squamous cell carcinoma. Am J Cancer Res. 2018;8(11):2296–2310.
- Zhang JJ, Wang DD, Du CX, et al. Long noncoding RNA ANRIL promotes cervical cancer development by acting as a sponge of miR-186. Oncol Res. 2018;26(3):345–352.
- Wu L, Zhang C, Chu M, et al. miR-125a suppresses malignancy of multiple myeloma by reducing the deubiquitinase USP5. J Cell Biochem. 2020;121(1):642–650.
- Liu Z, Zhang G, Yu W, et al. miR-186 inhibits cell proliferation in multiple myeloma by repressing Jagged1. Biochem Biophys Res Commun. 2016;469(3):692–697.
- Wu S, He X, Li M, et al. MiRNA-34a overexpression inhibits multiple myeloma cancer stem cell growth in mice by suppressing TGIF2. Am J Transl Res. 2016;8(12):5433–5443.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. 2014 International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Nihon Rinsho. 2016;74(Suppl 5):264–268.
- Tianhua Y, Dianqiu L, Xuanhe Z, et al. Long non-coding RNA Sox2 overlapping transcript (SOX2OT) promotes multiple myeloma progression via microRNA-143-3p/c-MET axis. J Cell Mol Med. 2020;24(9):5185–5194.
- Wang M, Zhao HY, Zhang JL, et al. Dysregulation of LncRNA ANRIL mediated by miR-411-3p inhibits the malignant proliferation and tumor stem cell like property of multiple myeloma via hypoxia-inducible factor 1alpha. Exp Cell Res. 2020;396(1):112280.
- Yang N, Chen J, Zhang H, et al. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017;8(8):e2975.
- Xu J, Su Y, Xu A, et al. miR-221/222-Mediated inhibition of autophagy promotes dexamethasone resistance in multiple myeloma. Mol Ther. 2019;27(3):559–570.
- Cao Y, Shi X, Liu Y, et al. MicroRNA-338-3p inhibits proliferation and promotes apoptosis of multiple myeloma cells through targeting cyclin-dependent kinase 4. Oncol Res. 2018;27(1):117–124.
- Anderson KC, Alsina M, Atanackovic D, et al. Multiple myeloma, Version 2.2016: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2015;13(11):1398–1435.
- Xiao X, Gu Y, Wang G, et al. c-Myc, RMRP, and miR-34a-5p form a positive-feedback loop to regulate cell proliferation and apoptosis in multiple myeloma. Int J Biol Macromol. 2019;122:526–537.
