ABSTRACT
Objectives
Changes in fecal microbiota affect the incidence and extent of graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Most patients with hematological malignancies receive antibiotics for the treatment of febrile neutropenia prior to allogeneic HSCT, and pre-transplant use of antibiotics may influence the fecal microbiota and GVHD.
Methods
We retrospectively analysed consecutive adult patients with hematological malignancies who received allogeneic HSCT at Chungnam National University Hospital between 2007 and 2018. Pre-transplant use of antibiotics was defined as the use of antibiotics before conditioning chemotherapy.
Results
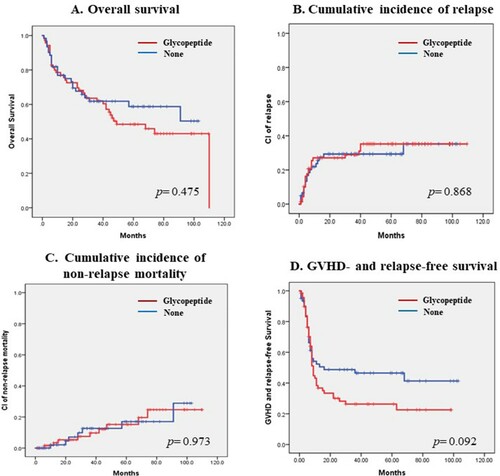
This study included 131 patients with a median age of 46 (range, 18–71) years: 76 (58%) patients were AML, 28 (21.4%) with ALL, 23 (17.6%) with MDS, and 4 (3.1%) with CML. All patients received calcineurin inhibitors with short-course methotrexate for GVHD prophylaxis. A total of 31 (23.7%) patients received anti-thymocyte globulin. All patients received antibiotics prior to HSCT: 70 (53.4%) patients received glycopeptide, 114 (87.0%) received cefepime, 87 (66.4%) received piperacillin/tazobactam, and 51 (38.9%) received carbapenem. Patients who received glycopeptide had more frequently extensive chronic GVHD (cGVHD) than those who did not (51.1% vs. 28.1% at 5 years) and had more frequently cGVHD of the lung (34.8% vs. 15.8% at 5 years). Pre-transplant use of glycopeptide did not affect the overall survival (OS) or GVHD- and relapse-free survival (GRFS) (median OS; 49 months in glycopeptide group vs. not reached in non-glycopeptide group, p=0.475; median GRFS; 9 months in glycopeptide group vs. 16 months in non-glycopeptide group, p=0.092).
Conclusion
Pre-transplant use of glycopeptide tends to increase the incidence of extensive cGVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is used to cure several hematological malignancies [Citation1]. However, graft-versus-host disease (GVHD) can cause fatal complications and decrease quality of life and is an obstacle for HSCT. Several factors such as donor type and human leukocyte antigen (HLA) disparity, the intensity of conditioning regimens, and whether immunosuppressants or anti-thymocyte globin (ATG) are used can affect the incidence and extent of acute and chronic GVHD [Citation2, Citation3].
Many studies have shown that the disruption of fecal microbiota during HSCT influences its outcomes in terms of GVHD [Citation4, Citation5]. For example, Weber et al. showed that decreased urinary excretion of 3-indoxyl sulfate (3-IS), the major conjugate of indole found in humans, reflects a disrupted microbiome [Citation6]. In that study, low 3-IS levels within the first 10 days after allogeneic HSCT were associated with significantly higher transplant-related mortality and worse overall survival. In addition, they mentioned that early initiation of antibiotic treatment was a risk factor for early suppression of 3-IS levels. Shono et al. also reported that the use of specific antibiotics for the treatment of febrile neutropenia during allogeneic HSCT, such as imipenem-cilastatin, was associated with GVHD-related mortality and changed the composition of stool microbiota in mouse models [Citation7].
We have used various antibiotics for the treatment of febrile neutropenia during remission induction or consolidation chemotherapy before allogeneic HSCT. The use of antibiotics prior to allogeneic HSCT could change the fecal microbiota environment and affect the outcomes of HSCT. However, no studies have explored this possibility, particularly in terms of GVHD for the patients with hematologic malignancies.
Methods
Patients
We conducted retrospective analyses of consecutive adult patients with hematological malignancies who received allogeneic HSCT at Chungnam National University Hospital between 2009 and 2018. We excluded patients who received haplo-identical donor or cord blood transplantation. Conditioning regimens were as follows. In the myeloablating conditioning (MAC) regimen, 3.2 mg/kg intravenous (IV) busulfan was administered for 4 days and 40 mg/m2 fludarabine was administered for 5 days (Bu4Flu) and 3.2 mg/kg IV busulfan was administered for 4 days and 60 mg/kg cyclophosphadmie was administered for 2 days (BuCy). In the reduced intensity conditioning (RIC) regimen, 3.2 mg/kg IV busulfan was administered for 2 days and 30 mg/m2 fludarabine was administered for 6 days (Bu2Flu). No pharmacokinetic adjustment of busulfan dose was performed. In HLA-matched sibling transplant, cyclosporine for GVHD prophylaxis was given commencing on day −1 with a target level of 150–300 ng/mL. In HLA-matched unrelated donor transplant, tacrolimus was given commencing on day −1 with a target level of 5–15 ng/mL. Short course of methotrexate was given on day +1, +3, +6 and +11 in all patients. Rabbit Anti-thymocyte globulin (thymoglobulin; Sanofi-Aventis, Paris, France) was given from days −3 to −1 at a dose of 1.5 mg/kg in some patients. Most of patients received granulocyte colony-stimulating factor-mobilized peripheral blood stem cells (PBSCs; target CD34+ cell count, 5 × 106/kg). Filgrastim 5ug/kg was administered from day +5 until neutrophil recovery. The study protocol was approved by the Institutional Review Board of Chungnam National University Hospital. The need for informed patient consent was waived given the retrospective nature of the analysis.
Endpoints
Primary endpoints were incidence and severity of acute and chronic GVHD according to the pre-transplant use of antibiotics. Secondary endpoints were overall survival, relapse rate, non-relapse mortality (NRM) and GVHD- and relapse-free survival (GRFS). Acute GVHD was graded according to the modified Seattle Glucksberg criteria and chronic GVHD was graded according to revised Seattle criteria [Citation8, Citation9]. NRM was defined as death from any cause other than relapse. The composite endpoint of GRFS was defined as extensive chronic GVHD, relapse or death.
Statistics
Categorical variables were compared using chi-square tests and logistic regression was used to evaluate the correlations. Survival was assessed using the Kaplan-Meier method. Survival rates were compared using the log-rank test. Multivariate analyses of independent prognostic factors for survival were performed using the Cox proportional hazard regression model with a 95% confidence interval. A p value < 0.05 was defined as significant. All statistical analyses were performed with the aid of SPSS software ver. 24.0 (IBM Corporation, Armonk, NY, U.S.A.).
Results
Patient characteristics
A total of 131 patients with a median age of 46 years (ranged from 18 to 71) were enrolled. The most common disease in these patients was acute myeloid leukemia (AML), followed by acute lymphoblastic leukemia (ALL) and myelodysplastic syndrome (MDS). About half of the patients received transplantation from sibling donors and the stem cell source was mostly mobilized peripheral blood hematopoietic stem cell. Most of patients (86.3%) received high-resolution DNA matched HLA-identical donor transplantation (eight out of eight alleles or ten out of ten alleles). 115 (87.8%) patients received myeloablating conditioning therapy, including Bu4Flu and BuCy. 31 (23.6%) patients received antithymocyte-globulin (ATG) for the prophylaxis of GVHD. 114 (87.0%), 87 (66.4%), 51 (38.9%) and 70 (53.4%) patients received cefepime, piperacillin/tazobactam, carbapenem and glycopeptide respectively, before allogeneic HSCT for the treatment of febrile neutropenia or infections. The median time from the last use of antibiotics to allogeneic HSCT was 49 days in cefepime, 73 days in piperacillin/tazobactam, 65 days in carbapenem and 60 days in glycopeptide, respectively. The median follow-up duration was 36 months, ranging from 1 to 110 months. shows patient demographics.
Table 1. Baseline characteristics of patients (N = 131).
Pre-transplant antibiotics and acute GVHD
There were no significant correlations between pre-transplant antibiotic usage and the incidence of acute GVHD (). Patients who received carbapenem and glycopeptide before allogeneic HSCT showed a slightly increased incidence of grade 3–4 acute GVHD compared to those who did not, but the difference was not statistically significant (grade 3–4 acute GVHD, carbapenem vs. no carbapenem, 9.8% vs. 2.5%, p=0.07; glycopeptide vs. not used, 7.1% vs. 3.3%, p=0.327)
Table 2. Correlation between pre-transplant antiobiotics and acute GVHD.
Pre-transplant antibiotics and chronic GVHD
Pre-transplant antibiotics did not affect any grade of chronic GVHD (). However, patients who received glycopeptide before allogeneic HSCT had more frequent extensive chronic GVHD than those who did not. The 5-year cumulative incidence of extensive chronic GVHD was 51.1% in the glycopeptide group, significantly higher than the 28.1% in the non-glycopeptide group (p=0.026, ). Interestingly, patients who received glycopeptide had more frequent chronic lung GVHD than those who did not. The 5-year cumulative incidence of chronic lung GVHD was 34.8% in the glycopeptide group and 15.8% in the non-glycopeptide group, with a p value of 0.028 (). Adverse risk factors for extensive chronic GVHD were the pre-transplant use of glycopeptide, HLA-mismatched donor, non-use of ATG, and grade 3–4 acute GVHD based on univariate analyses. However, in multivariate analyses, the poor prognostic factors that affected extensive chronic GVHD were mismatched donor, non-use of ATG, and severe acute GVHD (hazard ratio 2.2 [95% CI, 1.12–4.35], 3.73 [95% CI, 1.33–10.47], and 2.64 [95% CI, 1.66–4.19], respectively, ). And there was no significant risk factor in the multivariate analyses for chronic lung GVHD ().
Figure 1. (A-D). Cumulative incidence of extensive chronic GVHD according to pre-transplant use of antibiotics. (A) Cefepime, (B) Piperacillin/tazobactam, (C) Carbapenem, and (D) Glycopeptide.
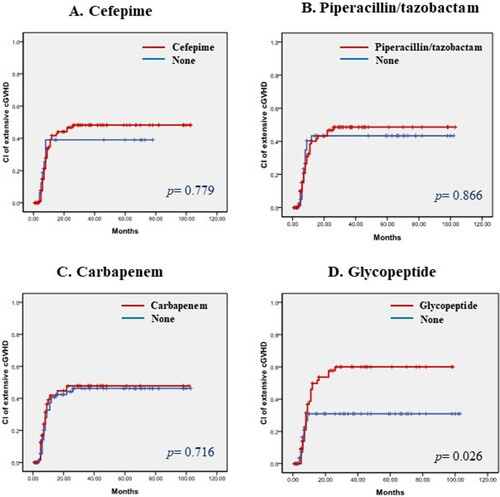
Figure 2. (A-D). Cumulative incidence of chronic lung GVHD according to pre-transplant use of antibiotics. (A) Cefepime, (B) Piperacillin/tazobactam, (C) Carbapenem, and (D) Glycopeptide.
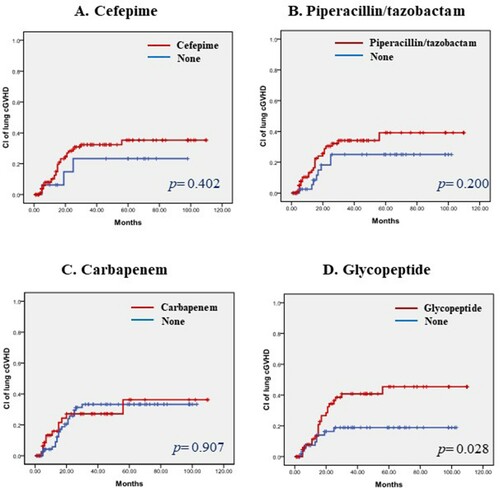
Table 3. Correlation between pre-transplant antiobiotics and chronic GVHD.
Table 4. Poor risk factors for extensive chronic GVHD in univariate and multivariate analysis.
Table 5. Poor risk factors for chronic lung GVHD in univariate and multivariate analysis.
Pre-transplant use of glycopeptide and survival
Patients who received glycopeptide before allogeneic HSCT had slightly decreased overall survival compared to those who did not, but the difference was not statistically significant (median OS; 49 months in glycopeptide group vs. not reached in non-glycopeptide group, p = 0.475, A). The cumulative incidence of relapse did not differ between the two groups (cumulative incidence of relapse at 5-yr; 35.2% in glycopeptide group vs. 29.3% in non-glycopeptide group, p = 0.868, B). Non-relapse mortality was not changed by pre-transplant use of glycopeptide (cumulative incidence of NRM at 5-yr; 19.6% in glycopeptide group vs. 18.8% in non-glycopeptide group, p = 0.973, C). Patients who received glycopeptide before allogeneic HSCT had slightly decreased GVHD- and relapse-free survival compared to those who did not, but the difference was not statistically significant (median GRFS; 9 months in glycopeptide group vs. 16 months in non-glycopeptide group, p = 0.092, D).
Discussion
We found that pre-transplant use of glycopeptide may be correlated with extensive chronic GVHD, particularly in the lung. In our study, those who used glycopeptide before allogeneic HSCT had an approximately two-fold higher 5-year cumulative incidence of extensive chronic GVHD than those who did not. This may be related to a disruption of gut microbiota. Microbiota play an important role in immune homeostasis, and disruption of the gut microbiota may affect immune reconstitution after allogeneic HSCT and could be correlated with acute and chronic GVHD [Citation10, Citation11]. For example, Chen et al. reported that changes in the intestinal microbiota composition are associated with acute GVHD [Citation12, Citation13]. In addition, Holler et al. showed that increases in the proportion of Enterococcus and decreases in commensal bacteria are common in patients who develop gastrointestinal GVHD [Citation14]. And, Jenq et al. reported that increased bacterial diversity is associated with reduced GVHD-related mortality [Citation15].
Most of patients with hematological malignancies commonly underwent broad-spectrum antibiotic therapy before allogeneic HSCT for the treatment of febrile neutropenia. Indeed, most patients were exposed to antibiotics before HSCT in our study, and these antibiotics might have affected the fecal microbiota [Citation16, Citation17]. Broad-spectrum antibiotics could not only reduce bacterial diversity but also increase resistant bacteria, and enable intrusion of pathogenic organisms through the depletion of natural intestinal microbiota. For example, Ferrer et al. showed that specific bacterial lineages, such as Enterococcus, Blautia, Faecalibacterium, and Akkermansia, are present in patients who receive β-lactam antibiotic intervention [Citation18]. And, Scott et al. showed that disruption of the gut microbiota by antibiotics reduces butyrate, which has protective effects against antibiotic-associated immune dysfunction in mice, in the intestine [Citation17, Citation19].
Fecal microbiota in patients with hematologic malignancies before allogeneic HSCT differs from that of the normal population. In the report of Kusakabe et al., populations of butyrate-producing bacteria were significantly lower in the fecal samples of patients before allogenic HSCT than in healthy controls. In addition, the population of Enterococcus was significantly higher in pre-transplant gut microbiotas [Citation20]. And, Peled et al. demonstrated that the diversity of intestinal microbiota before transplantation was quite variable, and a higher diversity at the time of neutrophil engraftment was associated with lower mortality [Citation21]. Glycopeptide is mainly used for the treatment of methicillin-resistant Staphylococcus aureus and pneumonia in febrile neutropenia during chemotherapy according to IDSA guidelines [Citation22, Citation23]. About half of our patients were treated with glycopeptide before allogeneic HSCT. This high usage rate of glycopeptide may have been due to the high rate of acute leukemia in our study population. Patients who had acute leukemia mostly experienced febrile neutropenia during the induction and consolidation chemotherapy. In this study, we showed the correlation between glycopeptide and inflammation of lung through the result that patients treated with glycopeptide had significantly increased extensive chronic GVHD of the lung. Some studies also have shown that vancomycin-induced gut dysbiosis during Pseudomonas aeruginosa pulmonary infection influences the lung-gut immunologic axis [Citation24]. However, this study did not reveal a specific mechanism, so further research is needed in the future for investigating the exact correlation between antibiotics and lung inflammation in patients underwent allogenic HSCT.
Pre-transplant use of glycopeptide increased the incidence of extensive chronic GVHD in univariate analyses in our study; however, the statistical power was weakened in multivariate analyses. This suggests that future studies with a larger number of patients are required. And a high-throughput molecular test for examining the composition of fecal microbiome is additionally needed to investigate whether pre-transplant use of glycopeptide actually changes the fecal microbiota.
In conclusion, this study shows the tendancy between pre-transplant antibiotic use and the incidence and severity of chronic GVHD in patients who underwent allogenic HSCT. We suggest that patients who are treated with glycopeptide prior to allogenic HSCT should be carefully monitored for the occurrence of extensive chronic GVHD.
Acknowledgements
We thank professor Deog Yeon Jo and Jaeyul Kwon for revising the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gooely TA, Chien JW, pergam SA, et al. Redued mortality after allogeneic hematopoietic cell transplantation. N Engl J Med. 2010;363:2091–2101.
- Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377:2565–2579.
- Dignan FL, Clark A, Amrolia P, et al. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158(1):30–45.
- Zama D, Biagi E, Masetti R, et al. Gut microbiota and hematopoietic stem cell transplantation: where do we stand? Bone Marrow Transplant. 2017;52(1):7–14.
- Andermann TM, Peled JU, Ho C, et al. The microbiome and hematopoietic cell transplantation: past, present, and future. Biol Blood Marrow Transplant. 2018;24(7):1322–1340.
- Weber D, Oefner PJ, Hiergeist A, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood. 2015;126(14):1723–1728.
- Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8(339):339ra71.
- Przepiorka D, Wisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
- Lee SJ, Vogelsan G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233.
- Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18(5):283–295.
- Peterson CT, Sharma V, Elmen L, et al. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015;179(3):363–377.
- Chen Y, Zhao Y, Cheng Q, et al. The role of intestinal microbiota in acute graft-versus-host disease. J Immunol Res. 2015;2015:145859.
- Taur Y, Jeng RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–1182.
- Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:640–645.
- Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:1373–1383.
- Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–4218.
- Scott NA, Andrusaite A, Andersen P, et al. Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci Transl Med. 2018;10(464):eaao4755.
- Ferrer M, Martins dos Santos VA, Ott SJ, et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut Microbes. 2014;5(1):64–70.
- Louis P, Scott KP, Duncan SH, et al. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102(5):1197–1208.
- Kusakabe S, Fukushima K, Maeda T, et al. Pre- and post-serial metagenomic analysis of gut microbiota as a prognostic factor in patients undergoing haematopoietic stem cell transplantation. Br J Haematol. 2020;188(3):438–449.
- Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382(9):822–834.
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious disease society of America. Clin Infect Dis. 2011;52(4):e56–e93.
- Lee DG, Kim SH, Kim SY, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2001;26(2):220–252.
- Rosa CP, Pereira JA, Cristina de Melo Santos N, et al. Vancomycin-induced gut dysbiosis during pseudomonas aeruginosa pulmonary infectionin a mice model. J Leukoc Biol. 2020;107(1):95–104.