ABSTRACT
Objectives
The present meta-analysis was performed to evaluate the efficacy, toxicities of both hypomethylating agents (decitabine and azaciticine) in the treatment of CMML patients.
Methods
All available cohort studies of patients with CMML treated with decitabine and azacitidine were identified. The primary endpoints of this meta-analysis were response to hypomethylating agents. Pooled estimates of treatment response and drug-related adverse events were calculated using fixed or random effect models.
Results
Fourteen studies with 600 CMML patients (decitabine: n=196; azacitidine: n=404) were identified and included for meta-analysis. HMAs yielded a pooled ORR estimate of 43% (95% CI: 36%-50%) in patients with CMML. Patients received either azacitidine or decitabine exhibited comparable incidence of ORR (43% vs. 45%, P=0.810), while significantly higher incidence of mCR was observed in patients treated with decitabine (23% vs. 10%, P=0.000). Decitabine treatment was also associated with higher incidence of transfusion independence (42% vs. 20%, P=0.044). Both HMAs led to objective hematologic or non-hematologic AEs (27%-43%), while dosage modification/delay were more frequent in patients treated with azacitidine (81% vs. 67%, P=0.021).
Conclusion
This current study may provide preliminary data in evaluating the efficacy and safety of HMAs in patients with CMML. Decitabine and azacitidine are comparable effective and safe in treating CMML. However, it is necessary to point out that any comparison of decitabine and azacitidine with respect to clinical outcomes can only be done in the context of a randomized controlled trial.
Introduction
Chronic myelomonocytic leukemia (CMML) is a heterogenous hematopoietic stem progenitor cells disorder that combines features of myelodysplastic syndromes and myeloproliferative neoplasm that occurs mostly in elder individuals [Citation1]. Treatment for CMML has been evolving from late 1990s. The major options for patients with CMML consist of chemotherapy such as cytarabine, etoposide, all-trans retinoic acid [Citation2,Citation3], 9-nitro-campothecin (topoisomerase inhibitor) [Citation4], topotecan [Citation5], and lonafarnib (farnesyltransferase inhibitor) [Citation6]. However, accompanied by disappointing response rate and frequent drug-related toxicities in these trials, outcome and survival of CMML are pessimistic. Hematopoietic stem cell transplant (HSCT) remains the only potentially curative treatment option for CMML, but is not suitable for most patients due to elder age and comorbidities.
While the hypomethylating agents have been prospectively shown to prolong overall surviall (OS) in MDS/CMML patients, only 14 CMML patients were included in each of these pivotal phase II/III randomized trials. Furthermore, response rates of these CMML patients were not reported separately [Citation7,Citation8]. Based on the outcome of these prospective trials with limited number of CMML, the United States Food and Drug Administration (FDA) had approved two hypomethylating agents, azacitidine and decitabine in treating patients with MDS/MPN. Since the publication of theses pivotal studies, reports of HMAs in treating CMML are still scarce. Most of these reports are either single arm trials or retrospective studies, while there is no existing randomized controlled trial showing a prolonged OS for HMA than BSC or other treatment in CMML by far.
This systematic review and meta-analysis were conducted with the aims to identify all publications reporting the outcomes of HMAs in treating patients with CMML, and further evaluate the efficacy and safety profile of HMAs in CMML patients.
Materials and methods
Data source
Databases of PubMed, Medline and EMBASE were screened for publishes in English between January 1996 and March 2020. Eligible studies were relevant clinical trials or retrospective cohort studies on CMML patients treated with hypomethylating regimens. Key terms used for searching were ‘chronic myelomonocytic leukemia’, ‘deicitabine’, ‘azacitidine’, ‘myelodysplastic synromes’ and ‘leukemia’. The references of eligible articles were also reviewed to identify additional studies. Principals for conducting this systematic review and meta-analysis is according to the ‘Preferred Reporting Items for Systematic Reviews and Meta-analysis’ [Citation9].
Study selection, inclusion criteria and data extraction
Identified publications were carefully screened. Preclinical studies, case reports, reviews and nursing care reports were excluded. For duplicate/overlap reports, only the latest updated report was included for meta-analysis. Studies were considered eligible for inclusion if they were (1) phase II–III clinical trials reporting outcomes of HMAs in CMML patients; (2) retrospective studies included at least 10 patients with CMML with detailed baseline information; (3) patients diagnosed with CMML according to the WHO (World Health Organization) classification; (4) treatment with single hypomethylating agent (decitabine or azacitidine) without other combination of chemotherapy, immunotherapy or hematopoietic stem cell transplantation; (5) reported at least one International Working Group (IWG) criteria based response rate, including overall response rate (ORR), complete response (CR), partial response (PR), marrow complete response(mCR) or hematologic improvement (HI). Two reviewers (Ruohao Xu and Minming Li) had screened all identified studies according to the including criteria. In total, 14 studies were chosen for the final analysis. (6) Abstracts published in conferences without a detailed report of patient outcomes were excluded from this systematic review and meta-analysis.
For data extraction, details including study characteristics, patient characteristics, treatment and follow-up information were extracted from selected studies. The primary endpoint of the meta-analysis was ORR (CR/PR/mCR/HI). CMML subtypes were stratified according to FAB [Citation10] or WHO [Citation11,Citation12] classification. As the 2016 revision of WHO Classification of Myeloid Neoplasms re-emphasizes the necessity of identifying the ‘proliferative’ (MP-CMML) or ‘myelodysplastic’ (MD-CMML) features according to a cutoff value of peripheral WBC > 13*109/l [Citation11], characteristics of these two subtypes were also extracted based on the peripheral WBC counts. Risk stratifications were documented according to the CMML-specific cytogenetic score (CPSS) [Citation13] or International Prognostic Scoring System (IPSS) [Citation14]. Grade 3 or higher adverse events (AEs) according to CTCAE (https://evs.nci.nih.gov/ftp1/CTCAE/About.html) were extracted for safety analysis, transfusion dependence and dosage modification or delay were also documented.
When extracting time-to-event data, we attempted to use primary values within the text of reports. When a study did not report details of this information in the text, digitizing software (Engauge Digitizer, http://digtizer.sourceforge.net) was used to extract data from the Kaplan-Meier curves in the original articles.
Statistical analysis
Pooled estimates of incidence of treatment response as well as adverse events were computed when sufficient data was acquired. Overall pooled effects assessment was conducted using a fixed – or random – effects model. Heterogeneity in the studies was assessed by χ2-test for heterogeneity and I2 measure of inconsistency. Heterogeneity was considered significant when P value of the Cochran Q test was <0.05, and the I2 statistic was classified as follows: 0–25% indicated insignificant heterogeneity; 26–50% indicated low heterogeneity; 51–75% indicated moderated heterogeneity; >75% indicated high heterogeneity. For effects assessment with moderate/high heterogeneity, random-effects models were used. For studies provided enough information, the odds ratio (OR) of response rate or hazard ratio (HR) of overall survival (OS) between subgroups was calculated. Both Begg’s and Egger’s tests were used to evaluate the publication bias regarding the primary endpoint ORR. A sensitivity analysis was also performed to describe the heterogeneity concerning the primary endpoint ORR. All statistical analysis was performed using the Meta-analysis program of STATA software (version 12.0).
Results
Study selection
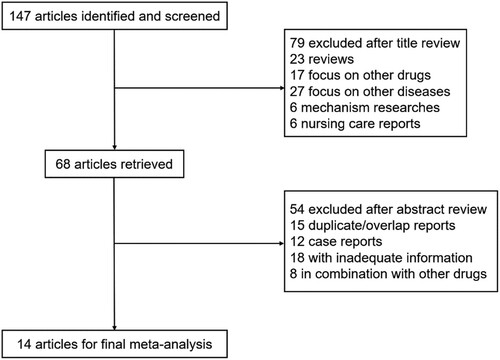
The present search strategy yielded a total of 147 studies for screening. Based on tittle and abstract review, 79 studies were irrelevant to the clinic use of hypomethylating agents in CMML patients and were excluded. Another 54 studies were eliminated due to overlapping reporting, case reporting, inadequate information and combination of other drugs. Thus, 14 clinical studies conducted between the years 1996 and 2019, which included 600 patients, fulfilled the inclusion criteria [Citation9] ().
Study characteristics
Characteristics of the 14 included publications were listed in and . Only five included studies were phase II clinical trials among the 14 publications: four studies were phase II, single armed trials of either decitabine or azacitidine in treating CMML [Citation15–18]; in another phase II trial, MDS and CMML patients were randomized to receive either azacitidine, azacitidine plus lenalidomide or azacitidine plus vorinostat in three groups [Citation19], outcomes of the single azacitidine treated CMML patients were extracted for meta-analysis. The author, research period, study type, drug regimen, dosing and median number of treatment cycles were listed in . As shown in , the median age ranged from 60 to 73.4 years, with 54.5 to 80% enrolled patients were male patients. The proportion of prior treated patients (chemotherapy, hydroxyurea or lenalidomide) varies from 0 to 55.6%. Among the 14 publications, 196 CMML patients were treated with decitabine from 5 studies, and 404 patients were treated with azacitidine from 10 studies, and one retrospective study reported outcomes of both HMAs [Citation20]. With the exception of study by Aribi et al. [Citation21], in which lower dose of decitabine (10 mg/m²) was used, other four decitabine based studies were conducted using a 15–20 mg/m² dosage, and all azacitidine based studies were conducted using a 75 or 100 mg/m² dosage. For effects assessment, all 14 included studies had applied the International Working Group (IWG 2006) response criteria [Citation22] for adverse events data to evaluate treatment response, 5 studies applied the Common Terminology Criteria for Adverse Events (CTCAE Criteria, https://evs.nci.nih.gov/ftp1/CTCAE/About.html), thus provided ideal effects assessment for pooling models.
Table 1. Characteristics of each study.
Table 2. Participant characteristics of included studies.
Publication bias and sensitivity analysis
No evidence of publication bias was detected for the primary endpoint ORR of this present study by either Egger test (P = 0.11) or Begg test (P = 0.11) (Supplemental Figure S1). To figure out the contribution of this heterogeneity, a sensitivity test was performed (Supplemental Figure S2).
Results of meta-analysis
Efficacy of hypomethylating agents in CMML patients
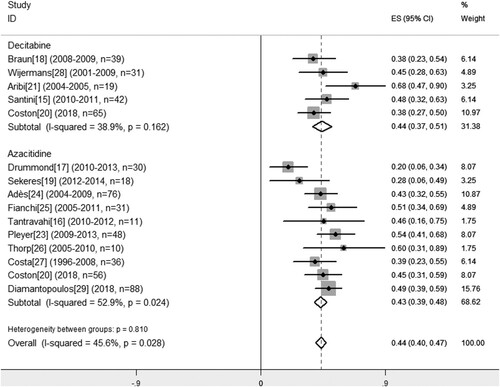
The endpoints of this meta-analysis were response to hypomethylating agents. The pooled estimates of response outcomes were listed in . Firstly, we calculated the pooled estimate of response rates to HMAs. Relative low heterogeneity for ORR across the 14 included studies was found according to the Cochran Q-test (P = 0.03, I2 = 45.6%). To figure out the contribution of this heterogeneity, a sensitivity test was performed, thus indicated acceptable heterogeneity from each included publication (Supplemental Figure S2). Thus, a fixed-effect model was employed to calculate the pooled estimate of ORR. As Cochran Q test indicating low heterogeneity concerning the PR rate (P = 0.088, I2 = 45.4%), a fixed-effect model was also used for calculating. As for the CR, mCR and HI rate with higher heterogeneity, a random-effect model was used. There were 6–14 studies available for a pooled estimate of ORR, CR, PR, mCR and HI event rates separately. Generally, hypomethylating agents yield a pooled ORR estimate of 44% (95% CI: 38–49%) in 14 studies with 600 CMML patients, with CR estimate of 16% (95% CI: 12–21%), mCR estimate of 15% (95% CI: 8–23%), PR of 3% (95% CI: 1–4%), and HI rate of 14% (95% CI: 10–16%) ().
Table 3. Meta-analysis and forest plots of overall response rate.
As for efficacy in subgroups concerning different HMAs, the pooled estimate of ORR was comparable in patients received either decitabine (45%; 95% CI: 36–55%) or azacitidine (43%; 95% CI: 36–50%) (P = 0.810) (). Among 7 studies with 367 CMML patients which the mCR data was available, patients treated with decitabine exhibited superior mCR rate (23%; 95% CI: 16–29%) than those patients treated with azaicitidine (10%; 95% CI: 3–18%) (P = 0.000). The pooled estimate of CR (18 vs. 16%, P = 0.553), PR (3 vs. 2%, P = 0.601) and HI (10 vs. 14%, P = 0.259) remained similar across included studies ().
Drug-related adverse events and transfusion-independence
It is reported that both decitabine and azacitidine could lead to high incidence of grade 3 or higher adverse events (AEs) during the treatment [Citation7,Citation8], most of which were hematopoietic associated effects. Among the 14 included publications, 7 studies employed [Citation15–18, 20,23,29] the CTCAE criteria to assess side-effects of decitabine or azacitidine. The pooled estimates of grade 3 or higher AEs are listed in . Because of the lower heterogeneity of the anemia, thrombopenia, and neutropenia rate, a fixed-effect model was employed to calculate these pooled estimates, while a random-effect model was used in calculating non-hematopoietic AEs due to a high heterogeneity. In general, models revealed that incidence AEs range from 23 to 35%, including anemia (31%; 95% CI: 23–38%), thrombopenia (35%; 95% CI: 28–42%), neutropenia (31%: 24–39%) and non-hematopoietic AEs (11%: 95% CI: 6–15%). Toxicity-related infectious event represents a subtype of non-hematopoietic adverse events, and may lead to serious consequences even death. Thus, we calculated the pooled estimates of toxicity-related infectious event to further investigated the risk for infection during the hypomethylating treatment. Five publications among the 14 included studies have reported incidence of toxicity-related infectious. The pooled estimates of grade 3 or higher infectious event were similar among patients treated with either azacitidine or decitabine (3 vs. 6%, P = 0.397), and the general estimate for HMAs was 6% (95% CI: 3–8%). As previously reported [Citation30,Citation31], intolerance to drugs or sever AEs could lead to dosage modifications and treatment delays of hypomethylating agents. Thus, we also observed high incidence of dosage modification/treatment delay (54%; 95% CI: 41–72%) using a random-effect model.
Table 4. Pooled estimates of drug-related adverse events and transfusion independence for hypomethylating agents.
Subgroup analysis between studies employed decitabine or azacitidine was performed. Similar to previous observation in MDS patients [Citation30,Citation32], we found higher incidence of thrombopenia (43 vs. 31%, P = 0.085) and non-hematopoietic AEs (19 vs. 8%, P = 0.035) in the decitabine group than in the azacitidine group, while the pooled estimates of anemia (31 vs. 30, P = 0.9), neutropenia (36 vs. 27%, P = 0.2) and infectious events (6 vs. 3%, P = 0.397) remained similar. Interestingly, our pooling model showed that the decitabine regimen significantly elevated the probability of transfusion independence (42 vs. 20%, P = 0.04) and lower incidence of dosage modification/delay (67 vs. 81%, P = 0.021) during the hypomethylating treatment, thus may be associated with alleviated need of RBC-transfusion. In most patients, the hematologic toxicity was transient and patients usually recovered before the next treatment cycle. Non-hematologic adverse events as vomiting, diarrhea and nausea were mild and reversible.
Predictive factors of hypomethylating agents in treating CMML
The prognosis of CMML patients is variable, with an approximate median survival of 2.5 years. Attempts had been made to establish factors with prognostic value in predicting response or OS after hypomethylating agents in CMML. These factors included bone marrow blasts (CMML 0/1/2) [Citation24], myeloproliferative features (increased WBC count, presence of splenomegaly), abnormal karyotypes, prior treatment and cytogenetic risk [Citation23,Citation24]. In order to verify factors with prognostic significance, we calculated the odds ratio (OR) of the ORR in CMML concerning baseline characters within each available study. These factors included WHO classification, myeloproliferative features, prior therapy and elder age (>65y) (). There was no difference in the incidence of ORR concerning WHO classification (OR: 0.926, P = 0.8), increased WBC counts (OR: 1.053, P = 0.89), splenomegaly (OR: 1.49, P = 0.79), karyotype (OR: 1.88, P = 0.18), elder age (OR: 0.72, P = 0.54) or patients gender (OR: 0.914, P = 0.86) (). Remarkably, our pooling model with 136 patients [Citation16,Citation18,Citation24,Citation26] indicated the ORR in pre-treated patients (chemotherapy, hydroxyurea or lenalidomide) was significantly higher than in those untreated patients (OR: 2.31, Z = 2.29, P = 0.02). Due to the lack of randomized trials and limited size of enrolled studies, this result concerning pre-treated patients should be interpreted with caution.
Table 5. Baseline factors associated with ORR.
Next, we tried to find out factors predicting OS after hypomethylating therapy. Survival analysis was performed based on studies provided details of subgroups [Citation18,Citation23,Citation26,Citation27], which consist of 130 individuals. Similar to our previous observation in MDS [Citation33], significantly prolonged OS in those HMA responders than non-responders was observed (HR: 2.791; 95% CI: 1.866–4.173; P = 0.00). Patients with myelodysplastic features (MD-CMML, WBC < 13*109/l) exhibited higher incidence of prolonged OS after hypomethylating therapy than those with proliferated features (MP-CMML, WBC > 13*109/l) (HR: 1.769; 95% CI: 1.195–2.62; P = 0.004) (). In the single study conducted by Adès et al. [Citation24], being one of the clinic features to distinguish myeloid proliferation, patients without splenomegaly showed higher incidence of longer OS (HR: 1.295; 95% CI: 0.735–2.357; P = 0.02). In another study conducted by Pleyer et al. [Citation23], patients without hydroxyurea treatment before azacitidine showed significant superior OS than those who received hydroxyurea before the hypomethylating treatment(HR: 2.385; 95% CI: 1.193–4.769; P = 0.011).
Table 6. Factors associated with longer OS.
Discussion
While the hypomethylating agents have been prospectively shown to prolong OS in MDS/CMML patients, only minor individuals with CMML are included in these pivotal phase II/III randomized trials [Citation7,Citation8]. Although both decitabine and azaicitidine have been approved by the US FDA, only the azacitidine regimen is approved by the European Medicine Agency for the treatment of non-proliferative CMML in Europe. In Italy, several national societies recommend HMAs should only be used in patients with myelodysplastic-type CMML and more than 10% bone marrow blasts [Citation34].
Data from phase II, single-armed trials and real-world studies indicate heterogeneous outcomes of HMAs in treating CMML: ORR ranges from 20 to 72%, 2-year survival from 25 to 48%, and median OS ranges from 7.1 to 37 months. Numbers of enrolled CMML patients in these studies are limited, most of which are consisted of less than 15 patients from single center, thus gives rise to the question of whether and in what depth could HMAs benefit CMML patients. Notably, there is still no evidence from randomized controlled trials showing a prolonged OS or higher ORR for HMAs than BSC or traditional regimens in CMML by far.
The present systematic review aimed to evaluate the efficacy and safety of two hypomethylating agents, decitabine and azaicitidine in treating patients with CMML. According to our pooling models, we found that HMAs yield a pooled ORR estimate of 44% (95% CI: 38–49%) in 14 studies with 600 CMML patients, with CR estimate of 16% (95% CI: 12–21%), mCR estimate of 15% (95% CI: 8–23%), PR of 3% (95% CI: 1–4%), and HI estimate of 14% (95% CI: 10–16%). Treatment of decitabine or azaicitidine was associated similar incidence of ORR (45 vs. 43%, P = 0.810). Patients treated with decitabine exhibit higher incidence of mCR (23 vs. 10%, P = 0.000), which is in consistent with previous reports comparing decitabine and azacitidine in MDS/CMML groups [Citation20,Citation30,Citation32,Citation33], indicating that decitabine may yield better response under certain circumstances.
With regard of the safety profile drug-related toxicities, the present pooling models yielded estimate of hematologic or non-hematologic AEs ranged from 23 to 35% of (). Several randomized phase II or retrospective studies comparing decitabine with azacitidine have reported a trend of higher incidence of hematologic toxicities in decitabine-treated groups [Citation30,Citation32,Citation35]. Similar to these findings, our analysis also revealed higher incidence of thrombopenia (43 vs. 31%, P = 0.085) and non-hematopoietic AEs (19 vs. 8%, P = 0.035) in patients treated with decitabine, while incidence of anemia and neutropenia remained similar between the two regimens. Interestingly, decitabine significantly alleviated the need of RBC-transfusion and dosage modification/delay in our analysis. This was contradicted with common notion that decitabine might lead to higher incidence of transfusion dependence in MDS/MPN [Citation32], thus larger cohorts of studies directly comparing the two HMAs in CMML are needed.
Outcomes concerning ORR and OS are heterogeneous in HMAs treated CMML patients. Although attempts had been made to combine predictive factors of response and survival in CMML, prognostic value of these factors are still controversial. Braun et al. [Citation18] finds that mutations in ASXL1, NRAS, KRAS, FLT3, CBL, and hypermethylation of the promoter of tumor suppressor gene-transcription-intermediary factor-1 (TIF1-y) fails to predict the ORR or OS after decitabine treatment in CMML patietns. Adès et al. [Citation24] shows that bone marrow blasts >10% and peripheral WBC > 13*109/l have a prognostic value on OS in CMML patients treated with azacitidine. In this present meta-analysis, we confirmed the prognostic value of several factors on OS, including CMML with myelodysplastic features (MD-CMML, WBC < 13*109/l), respondence to HMAs. Besides, we also found in our pooling model (with 136 CMML patients) that the ORR in pre-treated patients (chemotherapy or hydroxyurea) was significantly higher than in those untreated patients (OR: 2.31, Z = 2.29, P = 0.02). This result was in contrary to the common notion that pretreated patients often show poor outcome after hypomethylating therapy, thus this pooled estimate shall be interpreted with caution.
Weakness of our meta-analysis should not be ignored: firstly, only 5 of 14 included publications are phase II clinic studies, and no randomized controlled trials was enrolled. Results of these statistical analyses may lead to potential bias and confounding by including such observational studies. Secondly, as the there is no existing randomized controlled trial directly comparing the efficacy of decitabine and azacitidine in CMML, any comparison concerning clinical outcomes between HMAs can only be indirect. Thirdly, baseline characteristics of patients are likely to be different in the context of observational studies, differences in clinical outcomes could simply be a result of heterogenous patients characteristics. Thus, our pooled estimates can only serve as a preliminary data in evaluating the efficacy and safety of HMAs in CMML. Any definite conclusion should not be drawn before a randomized controlled trial be done.
Conclusion
To our knowledge, this study represents the first meta-analysis evaluating efficacy and safety profiles of HMAs in treating patients with CMML, as well as comparing efficacy of decitabine and azacitidine. As the there is no existing randomized controlled study reporting the efficacy of either HMA in a CMML cohort, this current systematic review and meta-analysis can only serve as a preliminary study in evaluating the efficacy and safety of HMAs in CMML. We provided evidences that both hypomethylating agents are effective and safe in treating CMML, while decitabine seems to bring with slightly higher incidence of treatment response and AEs under certain circumstances. At last, we strongly recommend that randomized controlled trials of HMAs in cohort of patients with CMML to be hold in the future.
Ethics approval and consent to participate
The need for ethics approval by an institutional board review was waived as this present study does not involve human subjects.
Availability of data and material
The datasets used and analyzed during the meta-analysis are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Patnaik MM, Tefferi A. Chronic myelomonocytic leukemia: 2018 update on diagnosis, risk stratification and management. Am J Hematol. 2018;93(6):824–840.
- Gerhartz HH, Marcus R, Delmer A, et al. A randomized phase II study of low-dose cytosine arabinoside (LD-AraC) plus granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in myelodysplastic syndromes (MDS) with a high risk of developing leukemia. EORTC Leukemia Cooperative Group. Leukemia. 1994;8(1):16–23.
- Cambier N, Wattel E, Menot ML, et al. All-trans retinoic acid in adult chronic myelomonocytic leukemia: results of a pilot study. Leukemia. 1996;10(7):1164–1167.
- Quintas-Cardama A, Kantarjian H, O'Brien S, et al. Activity of 9-nitro-camptothecin, an oral topoisomerase I inhibitor, in myelodysplastic syndrome and chronic myelomonocytic leukemia. Cancer. 2006;107(7):1525–1529.
- Beran M, Estey E, O'Brien S, et al. Topotecan and cytarabine is an active combination regimen in myelodysplastic syndromes and chronic myelomonocytic leukemia. J Clin Oncol. 1999;17(9):2819–2830.
- Feldman EJ, Cortes J, DeAngelo DJ, et al. On the use of lonafarnib in myelodysplastic syndrome and chronic myelomonocytic leukemia. Leukemia. 2008;22(9):1707–1711.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440.
- Kantarjian H, Issa J-PJ, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–1803.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. 2009;339:b2700.
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–199.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951.
- Such E, Germing U, Malcovati L, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. 2013;121(15):3005–3015.
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088.
- Santini V, Allione B, Zini G, et al. A phase II, multicentre trial of decitabine in higher-risk chronic myelomonocytic leukemia. Leukemia. 2018;32(2):413–418.
- Tantravahi SK, Szankasi P, Khorashad JS, et al. A phase II study of the efficacy, safety, and determinants of response to 5-azacitidine (Vidaza®) in patients with chronic myelomonocytic leukemia. Leuk Lymphoma. 2016;57(10):2441–2444.
- Drummond MW, Pocock C, Boissinot M, et al. A multi-centre phase 2 study of azacitidine in chronic myelomonocytic leukaemia. Leukemia. 2014;28(7):1570–1572.
- Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118(14):3824–3831.
- Sekeres MA, Othus M, List AF, et al. Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American Intergroup study SWOG S1117. J Clin Oncol. 2017;35(24):2745–2753.
- Coston T, Pophali P, Vallapureddy R, et al. Suboptimal response rates to hypomethylating agent therapy in chronic myelomonocytic leukemia; a single institutional study of 121 patients. Am J Hematol. 2019;94(7):767–779.
- Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109(4):713–717.
- Cheson BD. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425.
- Pleyer L, Germing U, Sperr WR, et al. Azacitidine in CMML: matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk Res. 2014;38(4):475–483.
- Ades L, Sekeres MA, Wolfromm A, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37(6):609–613.
- Fianchi L, Criscuolo M, Breccia M, et al. High rate of remissions in chronic myelomonocytic leukemia treated with 5-azacytidine: results of an Italian retrospective study. Leuk Lymphoma. 2013;54(3):658–661.
- Thorpe M, Montalvão A, Pierdomenico F, et al. Treatment of chronic myelomonocytic leukemia with 5-azacitidine: a case series and literature review. Leuk Res. 2012;36(8):1071–1073.
- Costa R, Abdulhaq H, Haq B, et al. Activity of azacitidine in chronic myelomonocytic leukemia. Cancer. 2011;117(12):2690–2696.
- Wijermans PW, Rüter B, Baer MR, et al. Efficacy of decitabine in the treatment of patients with chronic myelomonocytic leukemia (CMML). Leuk Res. 2008;32(4):587–591.
- Diamantopoulos PT, Kotsianidis I, Symeonidis A. Chronic myelomonocytic leukemia treated with 5-azacytidine – results from the Hellenic 5-Azacytidine Registry: proposal of a new risk stratification system. Leukemia Lymphoma. 2019;60(7):1721–1730.
- Lee YG, Kim I, Yoon SS, et al. Comparative analysis between azacitidine and decitabine for the treatment of myelodysplastic syndromes. Br J Haematol. 2013;161(3):339–347.
- Lee JH, Jang JH, Park J, et al. A prospective multicenter observational study of decitabine treatment in Korean patients with myelodysplastic syndrome. Haematologica. 2011;96(10):1441–1447.
- Jabbour E, Short NJ., Montalban-Bravo G, et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood. 2017;130(13):1514–1522.
- Xu R, Chen X, Deng C, et al. A retrospective study comparing azacitidine with decitabine in Chinese patients with refractory anemia with excess blast based on two clinical trials in a single center. Am J Transl Res. 2019;11(7):4533–4541.
- Onida F, Barosi G, Leone G, et al. Management recommendations for chronic myelomonocytic leukemia: consensus statements from the SIE, SIES, GITMO groups. Haematologica. 2013;98(9):1344–1352.
- Lee JH, Choi Y, Kim SD, et al. Comparison of 7-day azacitidine and 5-day decitabine for treating myelodysplastic syndrome. Ann Hematol. 2013;92(7):889–897.