ABSTRACT
Objective
HOTAIR has been well reported to be involved in the drug resistance of many diseases. This study aims to explore the possible implication of HOTAIR in doxorubicin (ADM) resistance in acute myeloid leukemia (AML).
Methods
Expressions of HOTAIR and PTEN in bone marrows of patient with newly diagnosed AML and relapsed/refractory AML and of healthy controls were determined by RT-qPCR. The half maximal inhibitory concentration (IC50) was calculated after AML-ADM-sensitive cells HL60 and AML-ADM-resistant cells HL60/ADM cells were treated by ADM. The IC50 of HL60/ADM to ADM dosage was determined by CCK-8. After cells were transfected with Sh-HOTAIR, pcDNA3.1-HOTAIR or pcDNA3.1-PTEN, cell biology of HL60/ADM cells was detected by flow cytometry, clone formation assay. The methylation of PTEN was determined by Methylmion-specific PCR and Bisulfite Genomic Sequence.
Results
Patient with relapsed/refractory AML had the highest HOTAIR and the lowest PTEN expression, followed by that in newly diagnosed AML patients and then healthy controls. After ADM treatment, cell viability and IC50 were enhanced in HL60/ADM cell when compared with HL60 cells. Up-regulated HOTAIR and down-regulated PTEN were found in HL60/ADM cells. Cell transfection with sh-HOTAIR or pcDNA3.1-PTEN leads to increased ADM sensitivity, apoptosis rate as well as decreased IC50 and cell clones, while those expression patterns can be reversed by co-transfection of pcDNA3.1-PTEN and pcDNA3.1-HOTAIR. Methylation was observed in the promoter of PTEN. HOTAIR can positively regulate DNMT3b.
Conclusion
HOTAIR suppresses PTEN through up-regulating DNMT3b-dependent way and confers ADM resistance in AML.
Introduction
Acute myeloid leukemia (AML), a subtype of acute leukemia, dominates the highest incidence in acute leukemia in adults with 20,000 newly diagnosed cases per year in United States alone [Citation1]. For patients with AML, the standard therapy strategy is allogeneic hematopoietic cell transplantation [Citation2,Citation3]. Admittedly, advancement and improvement on donor selection, supportive care and conditioning regimens lead to favorable outcomes for the prognosis of AML patients [Citation4]. Patients with hematological disorder, or exposure to topoisomerases II, alkylating agents or radiation are at high risk of AML [Citation5]. The well-accepted pathogenesis for AML is the uncontrolled proliferation and differentiation of myeloid stem cells within the bone marrow which was then infiltrated into peripheral blood and then organs [Citation1]. The past decades witnessed the progress in understanding the biology of this disease, how to use this knowledge to advance the development on new therapies for AML has been a great challenge. In addition, the understanding on disease pathology is currently focused on cellular and molecular level [Citation6], which encourages all the researchers to identify AML biomarkers, so as to facilitate early screening, diagnosis and treatment of AML.
Previous study identified neural cell adhesion molecule 1 (NCAM1) as an important biomarker for AML, considering its aberrant expression is associated with leukemic stem cells and stress resistance [Citation7]. Moreover, circular RNA (circRNA) circPAN3 was involved in adriacin doxorubicin (ADM) resistance in AML [Citation8]. Increasing studies highlighted that enhancing the AML sensitivity and suppressing the drug resistance are the two predominant factors in AML therapeutic development. HOX antisense intergenic RNA (HOTAIR) is a long non-coding RNA whose up-regulation has been shown to enhance estrogen receptor activity and consequently leads to tamoxifen resistance in breast cancer [Citation9,Citation10]. Aside from this, HOTAIR was also shown to promote hepatic insulin resistance in patients with type 2 diabetes mellitus via regulating SIRT1 [Citation11]. Additionally, HOTAIR is a well-proved biomarker for leukemia [Citation12,Citation13]. Although HOTAIR has been implicated in drug resistance in a wide range of diseases, no study assessed the possible link between HOTAIR and ADM resistance in AML.
Furthermore, another regulator in drug resistance, PTEN was also reported in insulin resistance and melanoma [Citation14,Citation15]. In addition to that, lncRNA HOTAIR/miR-17-5p/PTEN axis was suggested to have certain association with chemosensitivity of gastric cancer cells [Citation11]. Therefore, we speculate whether the interaction of HOTAIR and PTEN is still applicable in ADM resistance in AML. By measurement on acute myeloid leukemia-adriacin doxorubicin (AML-ADM) sensitive cells and AML-ADM resistant cells, we found methylation in the promoter of PTEN. The observation of current study showed that HOTAIR can activate PTEN methylation in a DNMT3b-dependent way, which consequently leads to ADM resistance in AML patients.
Materials and methods
Ethics statement
Experiments in this study were conducted with the approval from the ethics committee of Guangzhou First People’s Hospital. The informed consent was obtained from included patients or their family members.
Collection of bone marrow
From January 2018 to September 2019, a total of 19 cases of newly diagnosed AML patients and 11 cases of relapsed/refractory AML patients admitted to our hospital were included in this study. On parallel, 20 bone marrow samples from healthy donors were obtained as normal controls. Bone marrow mononuclear cells were separated from bone marrow using Ficoll-Pague lymphocyte separating medium.
Cell culture
Human AML-ADM-sensitive cells HL60 were purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA). AML-ADM-resistant cells HL60/ADM were obtained from Institute of Hematology, Chinese Academy of Medical Sciences (Tianjin, China). AML cells were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA) medium for incubation with a temperature of 37°C and 5% CO2. RPMI-1640 was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted by TRIzol (Invitrogen, Carlsbad, CA, USA). The reverse transcription reaction was performed using reverse transcription kit (TaKaRa, Tokyo, Japan). All operations were carried out in accordance with kit instructions. LightCycler 480 (Roche, Indianapolis, IN, USA) fluorescence quantitative PCR instrument was used to detect gene expression, and the reaction conditions were performed according to the requirements of the fluorescent quantitative PCR kit (SYBR Green Mix, Roche Diagnostics, Indianapolis, IN, USA). The thermal cycling parameters were as follows: 95°C for 10 s, followed by 45 cycles of 95°C for 5 s, 60°C for 10 s, 72°C for 10 s, the last, 72°C extension for 5 min. The quantitative PCR was set three repetitions for each reaction. U6 or glyceraldehyde phosphate dehydrogenase (GAPDH) was utilized as an internal reference. Data analysis was performed using the 2−ΔΔCt method, ΔΔCt = (Ct target gene−Ct internal reference) in the experimental group−(Ct target gene−Ct internal reference) in the control group. The sequences of amplification primers for each gene and its internal reference are shown in .
Table 1. Primer and sequences for quantitative reverse transcription polymerase chain reaction.
Cell counting kit-8 (CCK-8) assay
The half maximal inhibitory concentration (IC50) was calculated using CCK-8 assay. Digested cells were inoculated into a 96-well plate with 3000 cells/well before ADM of different concentrations (0, 0.1, 1, 2, 5, or 10 μg/mL) was supplemented for incubation of 24 h. Cells in each group were seeded into 3 different wells. Then 10 10 μL CCK-8 reagent (Tokyo, Dojindo, Japan) was added into each well for further incubation of 2 h before optical density (OD) value was measured at 450 nm.
Cell transfection
The Sh-HOTAIR and its negative control (NC, 2 μg), pcDNA3.1-Sh-HOTAIR and its NC (2 μg), pcDNA3.1-PTEN and its NC (2 μg) were obtained from Shanghai GenePharma Co., Ltd (Shanghai, China). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was used for cell transfection. About 24 h after cell transfection, cell apoptosis and clone formation ability were performed.
Flow cytometry
Cells in each group were washed in PBS for twice before cell suspension in 100 μL labeled solution for 10 min without light exposure. After that, 5 μL Annexin V-fluorescein isothiocyanate (FITC) and 5 μL propidium iodide (PI) were added for gently mixture with cells for 10 min reaction in a dark room. FITC fluorescence and PI fluorescence were detected by a Flow cytometer to calculate cell apoptosis rate. Three duplicates were set for each group.
Colony formation assay
Cells in each group were trypsinized and centrifuged at 25°C and 1500 rpm for 5 min before re-suspended in a complete culture medium. Cells (500 cells) in each group were seeded into 6-well plate, which contains a pre-warmed 2 mL complete culture medium for incubation at 37°C in a 5% CO2 incubator. The incubation was terminated once cell clones in the 6 well-plate were visible to the naked eyes. The plate was washed with PBS for twice with the culture medium discarded and then added 1.5 mL methanol to fix cells for 15 min. After that 1 mL Giemsa staining solution was added for staining for 20 min without light exposure. Then cells were washed with running water to wash away the residual staining solution and the inverted plate was dried in air before cell clones were calculated.
Methylmion-specific PCR (MSP)
DNA from cell genome was extracted using DNA extraction kit (Beyotime Biotechnology, Shanghai, China), as per the instructions. Then 3.6 M of Sodium bisulfite was prepared to modify DNA. The primers for MSP in PTEN promoter are listed in . The condition for MSP contains pre-denature at 94°C for 3 min, 35 cycles of 94°C for 30 s, 64 (58)°C for 1 min, 72°C for 45 s and a final extension at 72°C for 7 min. PCR products were subjected to agarose gel eletrophoresis before the brand was analyzed.
Table 2. Primers for methylmion-specific PCR.
Western blot
Cells were lysed in RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) to obtain the proteins. The protein concentration was determinated using bicinchoninic acid kit (Beyotime Biotechnology, Shanghai, China). Certain volume of proteins was mixed with loading buffer (Beyotime) for boiling water bath for 3 min before electrophoresis at 80 V for 30 min and 120 V for 1–2 h. The protein was transferred into a membrane in ice bath with a current of 300 mA for 60 min. After that, the membrane was washed in a washing buffer for 1 ∼ 2 min and then blocked for 60 min or at 4°C for overnight. Then primary antibody against GAPDH (5174S, 1:1000, Cell Signaling, Boston, USA), PTEN (9188, 1:1000, Cell Signaling, Boston, USA) was incubated with membrane for 1 h. After being washed for 3 × 10 min, membrane was probed with secondary antibody for incubation at room temperature for 1 h. The residual was washed for 3 × 10 min before the developer was added for color development. The analysis on brand was analyzed using chemiluminescence imaging system (Bio-Rad Laboratories, Hercules, CA, USA).
Bisulfite genomic sequence (BSP)
UCSC Genome Browser was applied to identify the PTEN CpG Island after that 0.5 mg genomic DNA was selected for sodium bisulfite treatment and PCR detection. The primers of PTEN are 5′-TTTGGTTTGTTTTT AGGGTAGTAGG-3′ and 5′-ATTAAAAAACTTTCCAA ATTCCCAC-3′. The BSP was performed with reference to a previous study [Citation16]. BSP determined the methylation rate of TA clone sequence, in which the CG sites are prone to methylation. In the case of methylation, the base sequence is expressed as CG, otherwise as TG. The methylation rate can be calculated based on the percentage of CG in total methylated sites.
Statistical analysis
SPSS 18.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6.0 (GraphPad Software Inc., La Jolla, CA, USA) were applied for data analysis. Measurement data were indicated as mean ± standard deviation. Comparisons between two groups were formulated by the t-test, while comparisons among multiple groups were assessed by one-way analysis of variance (ANOVA). The correlation between HOTAIR and PTEN expression in bone marrow samples was analyzed by Pearson correlation coefficient. P value less than 0.05 was indicative of statistically significant difference.
Results
AML patients had up-regulated HOTAIR and down-regulated PTEN
Bone marrow was collected from 30 AML patients and 20 healthy controls. The included AML patients were classified into newly diagnosed AML (n = 19) and relapsed/refractory AML (n = 11). The baseline characteristics of included AML patients are shown in . Ficoll lymphocyte separation fluid was used to separate bone marrow mononuclear cells. RT-qPCR was applied to determine the expressions of HOTAIR and PTEN in AML patients and healthy controls. The correlation of HOTAIR and PTEN was analyzed using Pearson correlation coefficient. The detection showed that newly diagnosed AML and relapsed/refractory AML patients had higher HOTAIR and lower PTEN than those in healthy controls ((A,B), P < 0.05). Newly diagnosed AML patients had down-regulated HOTAIR and up-regulated PTEN compared with those in relapsed/refractory AML patients ((A,B), P < 0.05). Analysis on correlation of HOTAIR and PTEN in bone marrow samples of 30 AML patients and 20 healthy controls showed that HOTAIR was negatively correlated with PTEN expression ((C), P < 0.05). Collectively, those data supported the implication of HOTAIR and PTEN in AML progression and drug resistance.
Figure 1. The expression of HOTAIR and PTEN in AML patients. RT-PCR was applied to measure the mRNA expressions of HOTAIR and PTEN in bone marrows of patients with AML and healthy controls. Compared with healthy controls, patients with AML had up-regulated expression of HOTAIR (A), but down-regulated expression of PTEN (B). The correlation analysis on HOTAIR and PTEN showed that HOTAIR expression was negatively correlated with PTEN (C). AML, acute myelocytic leukemia; HC, healthy controls; ND, patients with newly diagnosed AML; RE, patients with relapsed/refractory AML. *P < 0.05, **P < 0.01, n = 3.
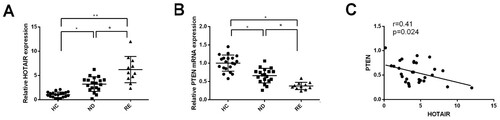
Table 3. Clinical data of AML patients.
AML-resistant cells had up-regulated HOTAIR and down-regulated PTEN
AML-sensitive cells H60 and AML-resistant cells HL60/ADM were treated by diluted ADM of various concentrations to verify the sensitivity or drug resistance of cells. CCK-8 assay was applied to measure the inhibitory rate and IC50 of ADM to H60 cells and HL60/ADM cells. The measurement showed that after ADM treatment, the cell viability of HL60/ADM cells was higher than those in H60 cells ((A), P < 0.05). Meanwhile, the IC50 of HL60/ADM to ADM was much higher than that in H60 cells ((B), P < 0.05). Those results showed that HL60/ADM cells were resistant to ADM treatment, while H60 cells were sensitive to ADM treatment. Therefore, HL60/ADM cells and H60 cells are applicable to further experiments.
Figure 2. AML-resistant cells had up-regulated HOTAIR and down-regulated PTEN. Up-regulated HOTAIR and down-regulated PTEN were found in AML-resistant cells. CCK-8 assay was applied to detect the cell viability of AML-sensitive cells HL60 and AML-resistant cells HL60/ADM after ADM treatment (A). IC50 was accordingly calculated (B) to verify the sensitivity and resistance of AML cells. qRT-PCR and Western blot were applied to measure the expressions of HOTAIR (C) and PTEN (D and E). IC50, half maximal inhibitory concentration; AML, acute myelocytic leukemia; ADM, adriacin doxorubicin. *P < 0.05, **P < 0.01, n = 3.
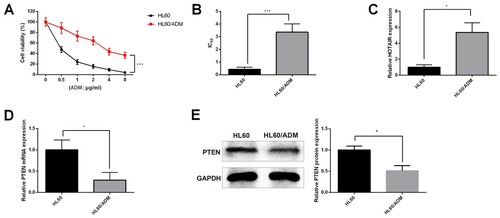
To further verify the effect of HOTAIR and PTEN to AML-resistance, RT-qPCR and Western blot were applied to detect the expressions of HOTAIR and PTEN in HL60/ADM cells and H60 cells. The detection showed that HOTAIR was highly expressed in HL60/ADM cells in comparison to that in H60 cells ((C), P < 0.05). Meanwhile, down-regulated PTEN was found in HL60/ADM cells when compared with that in H60 cells ((D,E), P < 0.05). Collectively, the expression pattern of HOTAIR and PTEN in AML-resistant cells and AML-sensitive cells was similar to that of newly diagnosed AML patients and relapsed/refractory patients. Taken together, HOTAIR and PTEN have certain roles to play in AML progression and are associated with ADM resistance.
Knockdown of HOTAIR ameliorates AMD resistance in HL60/ADM cells
HL60/ADM cells were transfected with sh-HOTAIR and received ADM treatment for 24 h later to detect the effect of HOTAIR on AMD resistance. Determination on HOTAIR by RT-qPCR showed that HOTAIR was significantly suppressed after sh-HOTAIR transfection ((A), P < 0.05). CCK-8 assay on IC50 showed that compared with the sh-NC group, the sh-HOTAIR group had much suppressed cell viability ((B), P < 0.05) and reduced IC50 ((C), P < 0.05). Then the cell apoptosis and proliferation of HL60/ADM cells were measured after sh-HOTAIR transfection and ADM treatment (2 μg/mL). The results showed that the sh-HOTAIR group exhibited increased cell apoptosis rate ((D), P < 0.05) and decreased cell clones ((E), P < 0.05) when compared with those in the sh-NC group. Collectively, knockdown of HOTAIR can enhance the sensitivity of HL60/ADM cells to ADM.
Figure 3. Knockdown of HOTAIR ameliorates AMD resistance in HL60/ADM cells. HL60/ADM cells were firstly transfected with sh-HOTAIR and pcDNA3.1-HOTAIR before qRT-PCR was applied to verify the transfection efficiency (A). CCK-8 assay showed that HOTAIR knockdown can suppress cell viability (B) and reduce IC50 (C). After cell transfection, 2 μg/mL ADM treatment was conducted for HL60/ADM cells. Flow cytometry and clone formation assay showed that knockdown of HOTAIR results in increased cell apoptosis (D) and suppressed cell clones (E). qRT-PCR and Western blot were applied to measure the expressions of PTEN (F and G). IC50, half maximal inhibitory concentration; AML, acute myelocytic leukemia; ADM, adriacin doxorubicin. *P < 0.05, **P < 0.01, n = 3.
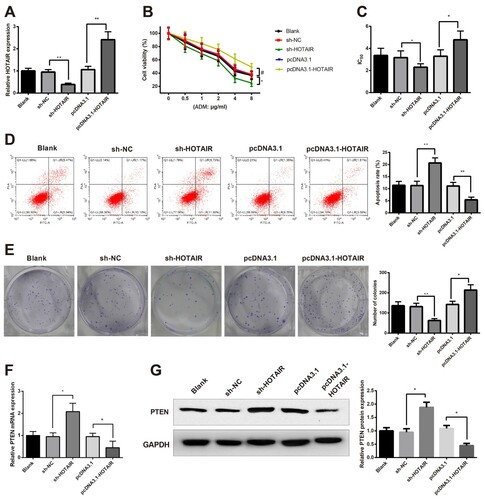
Meanwhile, we also identified the effect of HOTAIR knockdown on PTEN expression in HL60/ADM cells. Detection on PTEN demonstrated that sh-HOTAIR leads to elevated expression of PTEN ((F,G), P < 0.05). The results showed that HOTAIR can negatively regulate PTEN.
Overexpression of PTEN enhances the sensitivity of HL60/ADM cells to ADM
Dysregulation of PTEN was found in response to HOTAIR knockdown. Therefore, pcDNA3.1-PTEN or its negative control was transfected into HL60/ADM cells. RT-qPCR showed that PTEN expression was highly expressed in the pcDNA3.1-PTEN group when compared with the pcDNA3.1-NC ((A,B), P < 0.05). CCK-8 assay showed cell viability and IC50 were suppressed in the pcDNA3.1-PTEN group when compared with the pcDNA3.1-NC group ((C,D), P < 0.05).
Figure 4. Overexpression of PTEN enhances the sensitivity of HL60/ADM cells to ADM. HL60/ADM cells were transfected or co-transfected with pcDNA3.1-PTEN and pcDNA3.1-HOTAIR. Then qRT-PCR (A) and Western blot (B) showed mRNA expression of PTEN and protein expression of PTEN were elevated in response to transfection of pcDNA3.1-PTEN. Cell viability and IC50 were determined by CCK-8 (C and D) to measure the effect of PTEN on cell viability. The effect of PTEN on cell apoptosis was determined by Flow cytometry (E) and on cell proliferation ability was detected by Colony formation assay (F).AML, acute myelocytic leukemia; ADM, adriacin doxorubicin. *P < 0.05, **P < 0.01, n = 3.
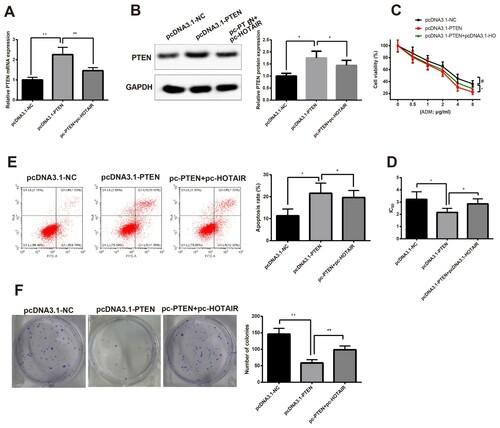
Then HL60/ADM cells were treated by 2 μg/mL of ADM for measurement of cell apoptosis and proliferation. Flow cytometry showed the pcDNA3.1-PTEN group had higher apoptosis rate and decreased cell clones in comparison to those in the pcDNA3.1-NC group ((E,F), P < 0.05). Co-transfection of PTEN and HOTAIR overexpression could reverse the suppressive effect of pcDNA3.1-PTEN in HL60/ADM cells. Taken together, overexpression of PTEN could enhance the sensitivity of HL60/ADM cells to ADM.
HOTAIR suppresses PTEN by up-regulating DNMT3b
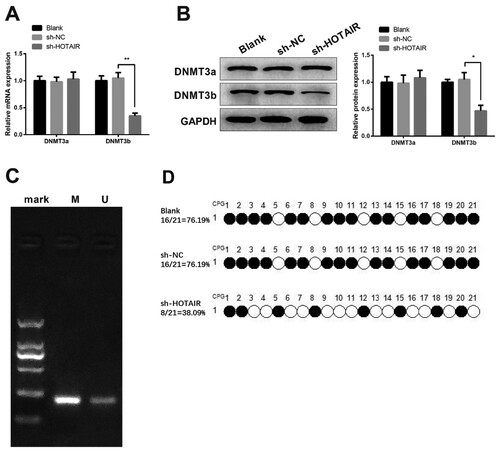
qRT-PCR and Western blot showed knockdown of HOTAIR leads to down-regulation of DNMT3b, while no significant difference in DNMT3a expression was observed ((A,B), P < 0.05). MSP showed hypermethylation of PTEN in HL60/ADM cells ((C), P < 0.05). BSP showed that compared with the blank group and sh-NC group, the methylation rate of PTEN is 76.19%, while the methylation rate in the sh-HOTAIR group is 38.09% ((D)).
Figure 5. HOTAIR can up-regulate DNMT3b to suppress PTEN. HL60/ADM cells were transfected with sh-HOTAIR before RT-qPCR (A) and Western blot (B) were used to measure the expressions of DNMT3a and DNMT3b. The methylation in promoter of PTEN was verified by MSP (C) and quantified by BSP (D). MSP, Methylmion-specific PCR; BSP, Bisulfite Genomic Sequence; ADM, adriacin doxorubicin. *P < 0.05, **P < 0.01, n = 3.
Discussion
To verify our hypothesis, we firstly measured the expression patterns of HOTAIR and PTEN in clinical bone marrow samples collected from both newly diagnosed AML and relapsed/refractory AML patients, as well as from healthy controls. Then this expression pattern, identified in clinical data, was verified in AML-ADM-resistant cells. To further excavate the possible effect of HOTAIR and PTEN in ADM resistance in AML cells, gain and loss of function was performed. At last, the methylation of PTEN and up-regulation of DNMT3b were observed in AML-ADM-resistant cells in response to HOTAIR knockdown. Collected data in this study showed that HOTAIR can activate the methylation of PTEN by up-regulating the expression of DNMT3b, thereby facilitating ADM resistance in AML.
Western blot and RT-qPCR were applied in clinical bone marrow samples and AML-ADM-resistant cell line, HL60/ADM. Observations demonstrated that HOTAIR was up-regulated, while PTEN was down-regulated in both AML patients and HL60/ADM cells, suggesting that HOTAIR and PTEN may have certain association with AML. As far as we know, chemo-resistance is a major contributor to the failure of drug treatment in a wide range of malignances, for instance, resistance to ADM is shown to discount the efficacy in chronic myelogenous leukemia [Citation17,Citation18]. We also identified AML patients with higher HOTAIR and lower PTEN expressions showed much stronger ADM resistance, evidenced by up-regulated HOTAIR and down-regulated PTEN in relapsed/refractory AML patients than those in newly diagnosed AML. The evidence suggested that the expressions of HOTAIR and PTEN may also associate with ADM resistance in AML. Further analysis on correlation showed that HOTAIR expression was negatively with that of PTEN, which indicate that HOTAIR may be directly or indirectly regulate PTEN in AML.
As mentioned above, no study reported the involvement of HOTAIR in ADM resistance in AML; therefore, we knockdown HOTAIR and overexpression PTEN in HL60/ADM to clarify their effect on ADM resistance. Detection on alternation of cell apoptosis and cell clone showed that knockdown of HOTAIR can enhance cell apoptosis but suppress cell clone in HL60/ADM. In this regard, it can be speculated that knockdown of HOTAIR can suppress ADM resistance and therefore enhance ADM sensitivity in HL60/ADM cells. HOTAIR is a well-known oncogenic lncRNA and a biomarker for poor prognosis in human cancers [Citation19–21]. Increasing evidence identified the possible association of elevated expression of HOTAIR with cisplatin resistance in various tumors, including breast cancer [Citation22] and in acute leukemia cells, K562/A02 [Citation23]. Several mechanisms have been proposed for the downstream targets of HOTAIR in regulating chemoresistance; however, limited data are available that can explain the up-regulation of HOTAIR in response to chemoresistance. In small cell lung cancer (SCLC), the expression of HOTAIR was regulated by H3K27me3 to affect the HOXA1 DNA methylation and SCLC chemoresistance [Citation24]. Moreover, we also found down-regulated PTEN expression in response to HOTAIR knockdown. Therefore, HOTAIR is able to regulate PTEN in HL60/ADM cells. Further detection on PTEN overexpression on ADM resistance showed that similar expression pattern to that of HOTAIR knockdown. However, we found PTEN overexpression cast no effect on HOTAIR in HL60/ADM cells, indicating PTEN is a downstream factor of HOTAIR. Partially consistent with our results, a previous study also demonstrated that silencing of HOTAIR can suppress AML progression via demethylation of HOXA5 by inhibiting DNMT3b [Citation25]. One previous study reported that HOTAIR by activating DNA methylation can suppress miR-122 expression in hepatocellular carcinoma [Citation26]. In addition to that, a research on chronic myeloid leukemia showed that advanced-staged patients had increased expression of increased HOTAIR and several DNA methyltransferases [Citation27]. Therefore, we speculate that HOTAIR maybe regulated PTEN in HL60/ADM cells by methylation.
MSP and BSP on HL60/ADM cells exhibited hypermethylation in the promoter of PTEN, which explained the down-regulated expression of PTEN in both AML patients and HL60/ADM cells. We also measured the expression of two methyltransferases in HL60/ADM cells with HOTAIR knockdown. The detection by qRT-PCR and Western blot showed that knockdown of HOTAIR leads to down-regulated expression of DNMT3b, while it has minimum effect on DNMT3a expression. DNA-Methyl-Transferases (DNMTs), including DNMT1, DNMT3b and DNMT3a, were frequently reported for their implication in AML [Citation28]. Functional experiments on embryonic stem cells showed that loss of DNMT3a or DNMT3b could result in the abolishment of methylation at corresponding enzyme-specific signatures [Citation29]. Different from DNMT3a, DNMT3b was revealed to regulate methylation in promoters of Sox9, Scleraxis, p21 and Bak1, so as to regulating cell death and cartilage [Citation30]. Collectively speaking, HOTAIR can induce methylation of PTEN by up-regulating DNMT3b.
Conclusion
Taken together, evidence in this study showed that HOTAIR can activate the methylation of PTEN so as to suppress the expression of PTEN in AML-ADM-resistant cells by up-regulating DNMT3b. Overexpression of HOTAIR confers drug resistance in AML. This study aims to provide theoretical basis for AML treatment by targeting the AMD resistance. However, the results of this study shall be interpreted with caution as this study has no relevant data concerning the possible downstream target for PTEN in ADM resistance in AML. Meanwhile, more evidence is required to explore the possible mechanism that is responsible for the regulation of HOTAIR in chemoresistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441.
- Kassim AA, Savani BN. Hematopoietic stem cell transplantation for acute myeloid leukemia: a review. Hematol Oncol Stem Cell Ther. 2017;10(4):245–251.
- Medinger M, Heim D, Halter JP, et al. Diagnostik und Therapie der Akuten Myeloischen Leukämie. Ther Umsch. 2019;76(9):481–486.
- Kadia TM, Ravandi F, Cortes J, et al. New drugs in acute myeloid leukemia. Ann Oncol. 2016;27(5):770–778.
- Sill H, Olipitz W, Zebisch A, et al. Therapy-related myeloid neoplasms: pathobiology and clinical characteristics. Br J Pharmacol. 2011;162(4):792–805.
- Prada-Arismendy J, Arroyave JC, Röthlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31(1):63–76.
- Sasca D, Szybinski J, Schüler A, et al. NCAM1 (CD56) promotes leukemogenesis and confers drug resistance in AML. Blood. 2019;133(21):2305–2319.
- Shang J, Chen WM, Liu S, et al. CircPAN3 contributes to drug resistance in acute myeloid leukemia through regulation of autophagy. Leuk Res. 2019;85:106198.
- Li Z, Qian J, Li J, et al. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3k/AKT/mTOR signaling pathway. Exp Ther Med. 2019;18(1):435–442.
- Xue X, Yang YA, Zhang A, et al. LncRNA HOTAIR enhances ER signaling and confers tamoxifen resistance in breast cancer. Oncogene. 2016;35(21):2746–2755.
- Li M, Guo Y, Wang XJ, et al. HOTAIR participates in hepatic insulin resistance via regulating SIRT1. Eur Rev Med Pharmacol Sci. 2018;22(22):7883–7890.
- Lin Y, Fang Z, Lin Z, et al. The prognostic impact of long noncoding RNA HOTAIR in leukemia and lymphoma: a meta-analysis. Hematology. 2018;23(9):600–607.
- Zhang YY, Huang SH, Zhou HR, et al. Role of HOTAIR in the diagnosis and prognosis of acute leukemia. Oncol Rep. 2016;36(6):3113–3122.
- Li A, Qiu M, Zhou H, et al. PTEN, insulin resistance and cancer. Curr Pharm Des. 2017;23(25):3667–3676.
- Zuo Q, Liu J, Huang L, et al. AXL/AKT axis mediated-resistance to BRAF inhibitor depends on PTEN status in melanoma. Oncogene. 2018;37(24):3275–3289.
- Ji W, Yang L, Yu L, et al. Epigenetic silencing of O6-methylguanine DNA methyltransferase gene in NiS-transformed cells. Carcinogenesis. 2008;29(6):1267–1275.
- Gao AM, Zhang XY, Ke ZP. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8(47):82085–82091.
- Ma X, Zhou X, Qu H, et al. TRIB2 knockdown as a regulator of chemotherapy resistance and proliferation via the ERK/STAT3 signaling pathway in human chronic myelogenous leukemia K562/ADM cells. Oncol Rep. 2018;39(4):1910–1918.
- Loewen G, Jayawickramarajah J, Zhuo Y, et al. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7:90.
- Qu X, Alsager S, Zhuo Y, et al. HOX transcript antisense RNA (HOTAIR) in cancer. Cancer Lett. 2019;454:90–97.
- Tang Q, Hann SS. HOTAIR: an oncogenic long non-coding RNA in human cancer. Cell Physiol Biochem. 2018;47(3):893–913.
- Wang Y, Wang H, Song T, et al. HOTAIR is a potential target for the treatment of cisplatinresistant ovarian cancer. Mol Med Rep. 2015;12(2):2211–2216.
- Li ML, Wang Y, Xu YN, et al. Overexpression of lncRNA-HOTAIR promotes chemoresistance in acute leukemia cells. Int J Clin Exp Pathol. 2020;13(12):3044–3051.
- Fang S, Shen Y, Chen B, et al. H3K27me3 induces multidrug resistance in small cell lung cancer by affecting HOXA1 DNA methylation via regulation of the lncRNA HOTAIR. Ann Transl Med. 2018;6(22):440.
- Wang SL, Huang Y, Su R, et al. Silencing long non-coding RNA HOTAIR exerts anti-oncogenic effect on human acute myeloid leukemia via demethylation of HOXA5 by inhibiting Dnmt3b. Cancer Cell Int. 2019;19:114.
- Cheng D, Deng J, Zhang B, et al. LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. EBioMedicine. 2018;36:159–170.
- Li Z, Luo J. Epigenetic regulation of HOTAIR in advanced chronic myeloid leukemia. Cancer Manag Res. 2018;10:5349–5362.
- Wong KK, Lawrie CH, Green TM. Oncogenic roles and inhibitors of DNMT1, DNMT3a, and DNMT3b in acute myeloid leukaemia. Biomark Insights. 2019;14:1177271919846454.
- Mao SQ, Cuesta SM, Tannahill D, et al. Genome-wide DNA methylation signatures are determined by DNMT3A/B sequence preferences. Biochemistry. 2020;59:2541–2550.
- Sanchez-Fernandez C, Lorda-Diez CI, Hurlé JM, et al. The methylation status of the embryonic limb skeletal progenitors determines their cell fate in chicken. Commun Biol. 2020;3(1):283.
