ABSTRACT
Background
Intermediate-risk acute myeloid leukemia (IR-AML) without FLT3-ITD, NPM1 and biallelic CEBPA mutations (here referred to as NPM1mut-negCEBPAdm-negFLT3-ITDneg AML) is a clinically heterogeneous disease. The optimal post-remission therapy (PRT) is unclear for patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML who achieved first complete response (CR1). This study aims to explore clinical and molecular factors that can help determine the prognosis of those patients and their choice of PRT.
Methods
We retrospectively analyzed 28 patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML who received induction chemotherapy and achieved CR1. For PRT, 17 patients received post-remission chemotherapy (PR-CT) and 11 patients received allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Results
For patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, multivariate analysis indicated that allo-HSCT and negative minimal residual disease (MRDneg) before PRT were favorable prognostic factors of overall survival (OS) (allo-HSCT, P = 0.002; MRDneg, P = 0.018); whereas relapse was an adverse prognostic factor of OS (P = 0.003). Log-rank analysis showed that allo-HSCT significantly improved their OS and RFS compared with PR-CT (OS, P < 0.001; RFS, P = 001). Otherwise, allo-HSCT improved the OS and RFS of patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, whether they obtained MRDpos or MRDneg before PRT (OS: MRDneg, P = 0.036; MRDpos, P = 0.012; RFS: MRDneg, P = 0.047; MRDpos, P = 0.030).
Conclusion
For patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, MRDneg before PRT and allo-HSCT were favorable prognostic factors of OS. Whether they obtain MRDneg or not, allo-HSCT is the preferred PRT.
Background
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by impaired differentiation and increased proliferation of myeloid progenitor cells [Citation1]. Although most patients with AML receive induction chemotherapy to achieve complete remission (CR), the relapse rate is still high and varies according to the cytogenetic and molecular profiles [Citation1]. Post-remission therapy (PRT) is applied to prevent relapse and usually includes 4–6 cycles of cytarabine, autologous hematopoietic stem cell transplantation (AHSCT), and allogeneic hematopoietic stem cell transplantation (allo-HSCT). In all PRTs, although allo-HSCT offers the strongest antileukemic effect, enhanced nonrelapse mortality (NRM) may compromise the benefit in terms of overall survival (OS). As a result, allo-HSCT is unsuitable for all patients with AML. The intensity and type of PRT is generally tailored according to risk profile. The European Leukemia Network (ELN) proposed three groups of standardized prognostic systems based on cytogenetics, molecular profiling, and clinical data [Citation1]. In general, allo-HSCT is recommended for poor risk and discouraged for good risk AML [Citation1,Citation2]. However, almost half of patients with AML are classified as intermediate-risk AML (IR-AML) determined by karyotype [Citation3]. For these patients, the best PRT is less clear and their prognosis is further determined by specific genetic changes, especially mutation in the nucleophosmin-1 gene (NPM1mut), biallelic mutation in the CCAAT/enhancer binding protein alpha gene (CEBPAdm), and mutation in the FMS-like tyrosine kinase 3 internal tandem duplication gene (FLT3-ITDmut) [Citation4–6].
In general, NPM1mut / FLT3-ITDmut-neg or CEBPAdm provides a good prognosis for IR-AML, whereas IR-AML with FLT3-ITDmut has a poor prognosis [Citation7–9]. For the subgroup of patients with IR-AML negative for NPM1mut, CEBPAdm, and FLT3-ITDmut (here referred to as NPM1mut-negCEBPAdm-negFLT3-ITDneg AML), the risk–benefit ratio of allo-HSCT is poorly defined. Schlenk et al. reported that the benefit of transplantation is limited for NPM1mut-negCEBPAdm-negFLT3-ITDneg AML [Citation7]. However, other retrospective studies reported a favorable OS after allo-HSCT for NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, and the survival is even similar to those of good risk group classified according to ENL criteria [Citation10,Citation11].
This study aims to explore additional clinical and molecular factors that can contribute to the prognosis of patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, and to assess the value of allo-HSCT in these patients.
Materials and methods
Patients
The study selected 122 newly diagnosed patients with AML who underwent therapy at Hainan Hospital of Chinese PLA General Hospital from July 2012 to March 2019. The last follow-up time was March 2020. All patients’ risk and prognosis groups were classified according to ENL criteria [Citation1].
Treatment protocols
Induction chemotherapy consisted of idarubicin 10 mg/m2/d for 3 days in combination with cytarabine 100–200 mg/m2/d for 7 days (IA); daunorubicin 60 mg/m2/d for 3 days in combination with cytarabine 100–200 mg/m2/d for 7 days (DA); mitoxantrone 10–12 mg/m2/d for 3 days in combination with cytarabine 100–200 mg/m2/d for 7 days (MA); or descitabine 20 mg/m2/d for 5 days in combination with aclacinomycin 10 mg/d for 1–5 days, cytarabine 10 mg/m2/12 h for 1–5 days, and G-CSF 5 µg/kg/d for 0–14 days (D-CAG). Patients who achieved partial remission (PR) were re-induced with the original scheme, whereas those who demonstrated no remission (NR) were induced with other schemes. Patients who achieved CR continued to receive 4–6 courses of consolidation chemotherapy with Ara-C (2–3 g/m2; every 12 h; days 1, 3, and 5) or proceeded to allo-HSCT. The choice of PRT depended on donor, individual wishes, finances, physical conditions, and genetic changes. Finally, a total of 11 patients underwent allo-HSCT, of which 5 and 6 underwent haploidentica HSCT and matched sibling HSCT, respectively. The transplantation scheme has been previously explained [Citation12,Citation13].
MRD, cytogenetics, and molecular analysis
The minimal residual disease (MRD) study was performed on bone marrow samples from patients who achieved CR. Collection and analysis were performed on a FACS Aria II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), which detected the immunophenotype of bone marrow cells through 14 - color immunomarker. Monoclonal antibody combinations used in all of the cases were as follows: CD34-FITC/CD13-PE/CDl17-PerCP/CD33-APC/HLA-DR-APC-CY7/CD45-V500 and CD38/CD7/CD56. The karyotype analysis of bone marrow samples was performed by g-banding method at initial diagnosis. Common fusion genes were detected by Prism 7500 real-time PCR. Target-region PCR enrichment and high-throughput parallel sequencing of gene mutations related to hematological malignancies were performed under the Ion Torrent sequencing platform. The average sequencing depth was 2000×, and the sensitivity was 1%. In total, 34 related gene mutations of AML/MDS/MPN were detected.
Endpoints and definitions
The endpoints include OS, which was measured from the date of diagnosis to death or the last follow-up time; relapse-free survival (RFS), which was measured from the date of CR1 to the first relapse, death or the last follow-up time; and cumulative incidence of relapse (CIR), which is defined as the ratio of the number of patients who have relapsed to the original total patients. Hematological CR was defined as less than 5% marrow blasts, no extramedullary disease, an absolute neutrophil count (ANC) of >1.0 × 109/L, a platelet count of >100 × 109/L, and independence from red cell transfusions. Relapse was defined as a recurrence of >5% bone marrow blasts, reappearance of blasts in the blood, or the development of extramedullary disease infiltrates at any site. Negative MRD before PRT (MRDneg) was defined as <10−3 blasts (<0.1%) in bone marrow samples. Positive MRD before PRT (MRDpos) was defined as ≥10−3 blasts (≥0.1%) in bone marrow samples. IR-AML without NPM1mut, CEBPAdm and FLT3-ITDmut was defined as NPM1mut-negCEBPAdm-negFLT3-ITDneg AML.
Statistical analysis
SPSS 20.0 was used for statistical analysis. Firstly, the normality test was conducted for all continuous variables, and mean ± standard deviation (SD) was used to describe the variables that followed normal distribution. Variables that don’t conform to normal distribution were described by median and quartile. Comparisons of patient characteristics between two groups were performed by independent-samples T test, Mann–Whitney U test, Chi-square or Fisher’s exact test. CRI was calculated by Kaplan-Meier method. Survival analysis was carried out by Kaplan-Meier method, and the difference between groups was compared by log-rank method. The factors affecting relapse was analyzed by Logistic analysis. The factors with P < 0.05 were entered into Cox regression model for multiple-factor analysis. Hazard ratios were presented with 95% confidence intervals (95% CI). A P value of <0.05 was considered to be statistically significant.
Results
Patients’ characteristics
According to karyotype, 64 patients were classified as intermediate-risk group (52.5%), 30 patients were classified as high-risk group (24.5%), and 28 patients were classified as low-risk group (23.0%). Among intermediate-risk group, 28 patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML (43.8%) who obtained CR within 2 course were further analyzed. We retrospectively analyzed their clinical features at diagnosis, including peripheral blood, white blood cell count (WBC), blast percentages in bone marrow and frequencies of known recurrent genetic mutations. No significant differences were found between PR-CT and allo-HSCT groups in terms of gender, WBC, bone marrow blasts and MRDpos or MRDneg. A lower percentage of one courses to CR, higher relapse rate and higher age were observed in the PR-CT group (P = 0.040, P = 0.042, P = 0.007, respectively) ().
Table 1. The characteristics of patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML.
Analysis of risk factors of OS, RFS, and relapse in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML
To assess the prognostic factors of patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML about OS, PFS, and relapse, we applied univariate and multivariate analysis in age (≥50 vs. <50 years), sex (male vs. female), WBC (≥100 vs. <100 × 109/L), blast (≥50% vs. <50%), courses to CR (>1 vs. 1 courses), MRD (positive vs. negative), treatment (PR-CT vs. allo-HSCT), and common genetic mutations (NRAS and C-Kit; mutated vs. wild). In patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, univariate analysis indicated that that relapse was an adverse factor of OS (P = 0.001), whereas allo-HSCT and MRDneg were favorable factors (P < 0.001, P = 0.043, respectively). allo-HSCT and MRDneg were favorable factors of PFS (P = 0.001, P = 0.030, respectively). No factors affected relapse. Multivariate analysis showed that allo-HSCT and MRDneg were independent favorable factors of OS (P = 0.002, P = 0.018, respectively), whereas relapse was independent adverse factor (P = 0.003). Allo-HSCT was independent favorable factor of RFS (P = 0.011) ().
Table 2. Univariate and multivariate analysis of OS, PFS and Relapse in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML.
Survival analysis in patients with IR-AML and NPM1mut-negCEBPAdm-negFLT3-ITDneg AML
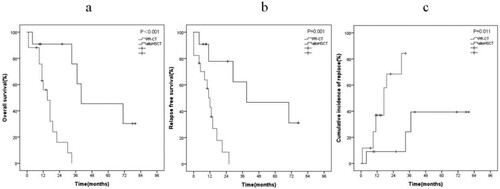
For patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, 11 patients (39.3%) received allo-HSCT, and 17 patients (60.7%) received PR-CT. Compared with PR-CT, allo-HSCT markedly improved the OS and RFS (OS, P < 0.001, (a); RFS, P = 0.001, (b)), and reduced the CIR (P = 0.011, (c)).
MRD analysis
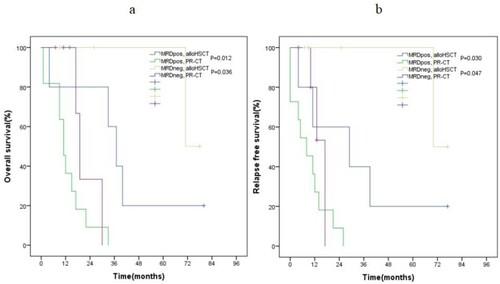
In our study, multivariate analysis showed that MRDneg was an independent favorable factor of OS in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML. Thus, we further performed a subgroup analysis. For patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, allo-HSCT also improved the OS and RFS of patients who obtained MRDpos or MRDneg compared with PR-CT (OS, P = 0.012, P = 0.036, respectively, (a); RFS, P = 0.030, P = 0.047, respectively, (b)).
Discussion
PRT in patients with AML includes continuing chemotherapy and AHSCT or allo-HSCT. In all PRT, although allo-HSCT has the strongest anti-leukemia effect, the benefits of OS is compromised by recurrent NRM, which limits its further application [Citation14]. For patients with AML in CR1, the indication for allo-HSCT is usually based on genetic risk factors. In general, patients with good cytogenetics receive PR-CT as preferred PRT, because the probability of obtaining a second CR is very high and the subsequent outcome upon proceeding to allo-HSCT in second CR is favorable [Citation15–17]. Allo-HSCT is considered to be the preferred PRT for patients with poor cytogenetics [Citation2]. However, for patients with IR-AML determined by karyotype, the best choice of PRT is not clear [Citation18,Citation19].
With the development of second-generation sequencing technology, the role of molecular genetics in prognostic assessment has been increasingly emphasized in the past decade to improve prognostic stratification, especially for IR-AML [Citation20]. For example, IR-AML with FLT3-ITDmut has poor prognosis in terms of increasing the risk of relapse and death. Compared with PR-CT, allo-HSCT reduces the risk of relapse and improves RFS and OS in this group [Citation9]. IR-AML with NPM1mut/FLT3-ITDmut-neg or CEBPAdm usually has good prognosis, high CR rate, and high survival rate [Citation7,Citation8]. In this group, procedure-related mortality after allo-HSCT offsets disease benefits. The standard treatment for this group is induction chemotherapy followed by three or four high-dose cytarabine consolidation cycles [Citation7,Citation21]. A remaining issue is the poorly defined risk–benefit ratio of allo-HSCT in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML. Data comparing the outcomes of allo-HSCT and PR-CT for NPM1mut-negCEBPAdm-negFLT3-ITDneg AML are few and unclear. Schlenk et al. reported that the benefit of transplantation is limited for NPM1mut-negCEBPAdm-negFLT3-ITDneg AML [Citation7]. Heidrich et al. demonstrated that patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML can achieve longer RFS from matched sibling allo-HSCT compared with PR-CT [Citation11]. Ying Zhang et al. indicated a 5-year OS close to 59% for NPM1mut-negCEBPAdm-negFLT3-ITDneg AML patients in allo-HSCT group, whereas 33% in PR-CT (P = 0.024) [Citation22]. In our study, multivariate analysis showed that allo-HSCT was an independent good prognostic factor (P = 0.002) and improved the OS and RFS compared with PR-CT (OS, P < 0.001, (a); RFS, P = 0.001, (b)) in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML. We believe that the improvement in survival is mainly due to the lower risk of relapse after allo-HSCT, whereas the CIR of patients treated with allo-HSCT is better than that with PR-CT (P = 0.011, (c)). Thus, for patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, allo-HSCT is also a preferred PRT over PR-CT. Although the patients treated by allo-HSCT was younger and had a lower percentage of one courses to CR, multivariate analysis showed that the age and courses to CR weren’t independent prognostic factors for patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML. So the effect of age and courses to CR on the reliability of the result were little.
The recent studies showed that combining MRD with treatment-related prognosis will contribute to favorable clinical outcomes. For example, Ying Zhang et al. demonstrated that allo-HSCT and MRDneg were independent favorable prognostic factors of OS and RFS in patients with IR-AML [Citation22]. Ravandi et al. confirmed that MRDpos was an independent adverse prognostic factor of OS and RFS in patients with AML [Citation23]. Our results are similar to the previous studies. In our study, besides allo-HSCT, multivariate analysis showed that MRDneg is also an independently favorable prognostic factor of OS in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML (P = 0.018). Based on the above results, we assumed that patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML who obtained MRDneg may not need allo-HSCT. The subgroup analysis showed that, although MRDneg was a predictor for better OS in patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, allo-HSCT also improved their OS and RFS compared with PR-CT (OS, P = 0.036 (a); RFS, P = 0.047 (b)). Similar to patents with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML who obtained MRDneg, allo-HSCT also improved the OS and RFS of patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML who obtained MRDpos compared with PR-CT (OS, P = 0.012 (a); RFS, P = 0.030, (b)). Thus, we suggest that whether or not patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML obtained MRDneg, allo-HSCT is a preferred PRT over PR-CT.
Our work has some limitations. First, this work was a retrospective study that resulted in a lack of molecular profiling data in a proportion of patients. Second, our study involved few patients. Therefore, caution should be taken when interpreting our data given that this work was a single-center, retrospective study with a small number of heterogeneous patients.
In summary, for patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML, MRDneg and allo-HSCT were favorable prognostic factors and relapse was risk prognostic factor of OS. Our results found that allo-HSCT is a preferred PRT for patients with NPM1mut-negCEBPAdm-negFLT3-ITDneg AML over PR-CT, even if these patients obtained MRDneg.
Ethics approval and consent to participate
The ethics committee waived the need for informed consent for this retrospective study because of the absence of impact on the management of patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447.
- Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–590.
- Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365.
- Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML study group Ulm. Blood. 2002;100(13):4372–4380.
- Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089.
- Ostronoff F, Othus M, Lazenby M, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol. 2015;33(10):1157–1164.
- Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008; 358(18):1909–1918.
- Marcucci G, Maharry K, Radmacher MD, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: a Cancer and Leukemia Group B study. J Clin Oncol. 2008;26(31):5078–5087.
- Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–741.
- Ahn JS, Kim HJ, Kim YK, et al. Transplant outcomes of the triple negative NPM1/FLT3-ITD/CEBPA mutation subgroup are equivalent to those of the favourable ELN risk group, but significantly better than the intermediate-I risk group after allogeneic transplant in normal-karyotype AML. Ann Hematol. 2016;95(4):625–635.
- Heidrich K, Thiede C, Schafer-Eckart K, et al. Allogeneic hematopoietic cell transplantation in intermediate risk acute myeloid leukemia negative for FLT3-ITD, NPM1- or biallelic CEBPA mutations. Ann Oncol. 2017;28(11):2793–2798.
- Zhang WP, Yang D, Song XM, et al. Allogeneic peripheral blood stem cell transplantation is a promising and safe choice for the treatment of refractory/relapsed acute myelogenous leukemia, even with a higher leukemia burden. Biol Blood Marrow Transpl. 2013;19(4):653–660.
- Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transpl. 2006;38(4):291–297.
- Cornelissen JJ, Breems D, van Putten WL, et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol. 2012;30(17):2140–2146.
- Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013;31(10):1293–1301.
- Schlenk RF, Taskesen E, van Norden Y, et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood. 2013;122(9):1576–1582.
- Jourdan E, Boissel N, Chevret S, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. 2013;121(12):2213–2223.
- Cornelissen JJ, Versluis J, Passweg JR, et al. Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40–60 years. Leukemia. 2015;29(5):1041–1050.
- Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127(1):62–70.
- Sanders MA, Valk PJ. The evolving molecular genetic landscape in acute myeloid leukaemia. Curr Opin Hematol. 2013;20(2):79–85.
- Rollig C, Bornhauser M, Kramer M, et al. Allogeneic stem-cell transplantation in patients with NPM1-mutated acute myeloid leukemia: results from a prospective donor versus no-donor analysis of patients after upfront HLA typing within the SAL-AML 2003 trial. J Clin Oncol. 2015;33(5):403–410.
- Zhang Y, Zhang Y, Chen Q, et al. Allogeneic hematopoietic stem cells transplantation improves the survival of intermediate-risk acute myeloid leukemia patients aged less than 60 years. Ann Hematol. 2019;98(4):997–1007.
- Ravandi F, Jorgensen J, Borthakur G, et al. Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer. 2017;123(3):426–435.