ABSTRACT
Fms-like tyrosine kinase 3 (FLT3) is one of the most frequently mutated genes in acute myelogenous leukemia (AML) and the mutation is associated with poor prognosis of patients. Two distinct types of activating mutations have been identified in AML samples. One is internal tandem duplications in the juxtamembrane domain (FLT3-ITD) and the other is point mutations in the tyrosine kinase domain (FLT3-TKD). Gilteritinib is a FLT3 inhibitor that inhibits both FLT3-ITD and FLT3-TKD. It was reported that differentiation of leukemic blasts accompanied by differentiation syndrome occurs in some patients treated with gilteritinib. However, information about the precise clinical course is limited, and appropriate management of differentiation syndrome has not been established. We report a case of relapsed AML with FLT3-ITD that was treated with gilteritinib. Analysis of the FLT3-ITD variant allele frequency (VAF) revealed that FLT3-ITD VAF was not decreased despite achievement of complete remission with incomplete hematologic recovery. Remarkable increases of monocytes and granulocytes accompanied by differentiation syndrome were observed at 6 months after the initiation of gilteritinib treatment. Intermittent chemotherapy with low-dose cytarabine and mitoxantrone was effective for reducing myelomonocytosis and resolving differentiation syndrome.
Introduction
Fms-like tyrosine kinase 3 (FLT3) encodes a receptor-type tyrosine kinase, and two distinct types of activating mutations have been identified in AML samples. One is internal tandem duplications in the juxtamembrane domain (FLT3-ITD) and the other is point mutations in the tyrosine kinase domain (FLT3-TKD). It has been established that FLT3-ITD mutation in AML with a normal karyotype is associated with poor prognosis. Identification of FLT3-ITD mutation is important not only for risk stratification of patients with AML based on the genetic status of FLT3 but also for development of a molecular targeting drug [Citation1].
Gilteritinib is a FLT3 inhibitor that inhibits both FLT3-ITD and FLT3-TKD and it is effective for relapsed/refractory AML (R/R-AML) with FLT3 mutations. Since FLT3 is one of the most frequently mutated genes in AML, the use of gilteritinib seems to be increasing [Citation2,Citation3]. It was reported that differentiation of leukemic blasts accompanied by differentiation syndrome (DS) occurs in 3% of patients treated with gilteritinib [Citation4,Citation5]; however, detailed information about DS is limited. Here, we report a case of R/R-AML with FLT3-ITD in which myelomonocytic differentiation of leukemia cells was provoked and DS was observed at 6 months after the initiation of gilteritinib treatment.
Case report
An 81-year-old man presented with a 1-month history of general fatigue. Blood examination revealed peripheral leukocytosis (23.1 × 109/L) accompanied by moderate anemia (hemoglobin, 82 g/L) and mild thrombocytopenia (platelet count, 100 × 109/L). Hepatosplenomegaly was not observed. Bone marrow examination showed normocellular marrow with 52.0% blasts, which were positive for myeloperoxidase and non-specific esterase activity. Karyotype analysis revealed a normal karyotype with 24 metaphase. Mutational analysis for nucleophosmin 1 (NPM1) and CEBPA was performed by polymerase chain reaction (PCR) amplification followed by Sanger sequencing. An insertion of TCTG between nucleotides 860 and 863 (c.863_864insTCTG) in NPM1 was detected. On the other hand, no mutations were detected in CEBPA. FLT3 was analyzed for internal tandem duplication (ITD) by PCR amplification followed by gel electrophoresis, for which the sensitivity of detection is recognized as being about 5% FLT3-ITD variant allele frequency (VAF) [Citation6]. The patient was diagnosed as having AML with mutated NPM1 according to the WHO classification. Low-dose chemotherapy by the CAG regimen (low-dose cytarabine, aclarubicin and granulocyte colony-stimulating factor) [Citation7] was performed and he achieved complete remission (CR) after two cycles. Thereafter, azacitidine maintenance therapy was introduced [Citation8]; however, the patient relapsed with leukocytosis (41.1 × 109/L) 12 months after the onset of AML. Bone marrow examination showed hypercellular marrow with 85.0% blasts. Genetic status was re-evaluated, and FLT3-ITD mutation, in addition to NPM1 mutation, was detected in relapsed AML cells (a). Although re-induction chemotherapies (CAG therapy, gemtuzumab ozogamicin and low-dose cytarabine) were attempted [Citation9,Citation10], remission was not obtained. Gilteritinib was approved for clinical use and became available in Japan 7 months after the relapse of AML. Therefore, gilteritinib monotherapy was initiated at a dose of 120 mg just after its approval for clinical use (). Although leukemic blasts remained but were decreased in bone marrow at day 20 (), they disappeared quickly from peripheral blood. Thereafter, he was basically followed on an outpatient basis. He required blood transfusion of both red blood cells and platelets weekly in the second month; however, transfusion dependency increased in the third month. Bone marrow examination at day 76 showed that CR with incomplete hematologic recovery (CRi) had been achieved (). Since a gastrointestinal tract examination revealed that extensive erosion was the main reason for anemia, possibly due to an adverse event caused by gilteritinib, dose reduction and interruption of gilteritinib administration were conducted. After recovery from gastrointestinal bleeding, gilteritinib was re-administered at a dose of 80 mg. Hematopoiesis gradually increased and he became transfusion-independent after day 159. However, white blood cells (WBC) continued to increase with monocytosis. Significant leukocytosis with myelomonocytosis was observed after day 159, while a bone marrow examination at day 169 showed proliferation of leukemic blasts ((b and c), ). Initially, we thought that a dose of 80 mg of gilteritinib was insufficient to inhibit proliferation of leukemic blasts, and we increased the dose of gilteritinib to 200 mg. However, progression of anemia was observed by recurrence of melena, although WBC decreased. Therefore, we stopped administration of gilteritinib and administered low-dose cytarabine. Body weight gain and slight dyspnea were observed around day 180, but these symptoms were resolved along with decrease of leukocytosis. After transient suppression of leukocytosis, WBC increased maximally to 140 × 106/mL with 54% of monocytes at day 217. We assumed that the increased numbers of both granulocytes and monocytes in peripheral blood were differentiated from leukemic blasts. At that point, fever (37–38°C), peripheral edema, elevated serum creatinine (1.53 mg/dL), body weight gain (from 51.7 to 61.2 kg) and dyspnea with interstitial pulmonary infiltrates were simultaneously observed. Pulmonary congestion, increased vascular pedicle width and enlarged heart were observed by a chest X-ray (). Although these symptoms were suspected to be consistent with DS [Citation11], we could not confirm the differentiation of leukemic blasts because the onset of DS was 6 months after the initiation of gilteritinib treatment and evaluation of FLT3-ITD VAF was not performed at that time. Since the reduction of the leukemic cell burden by 200 mg gilteritinib and low-dose cytarabine treatment was associated with relief from body weight gain and dyspnea, we thought that leukemic blasts were still responsive to gilteritinib and that increased myelomonocytes in peripheral blood might be the main cause of DS. Thus we treated the patient with low-dose cytarabine and mitoxantrone. This treatment resolved DS and induced a stable condition of the disease in which leukemic blasts had disappeared from peripheral blood with sustained leukemic blasts in bone marrow. This condition was maintained by resumption of gilteritinib treatment ().
Figure 1. Smears of bone marrow and peripheral blood. (a) Bone marrow aspirates at relapse showed proliferation of blasts. (b) Bone marrow aspirates at day 169 showed proliferation of blasts with promonocytic differentiation. (c) Increase of abnormal monocytes with convoluted or folded nuclei in peripheral blood smears at day 169.
Figure 2. Clinical course. Gilteritinib was orally administered at the indicated dose once a day. Low-dose cytarabine (16 mg) was subcutaneously injected twice a day for 7 days. Mitoxantrone was intravenously administered once at a dose of 5 mg.
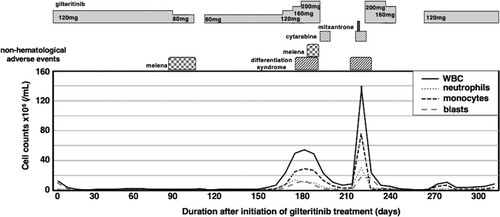
Figure 3. Adverse events caused by gilteritinib. Chest X-ray at the time of differentiation syndrome showed bilateral infiltrates and an enlarged cardiac silhouette (right panel). Left panel shows a chest X-ray before the onset of differentiation syndrome.
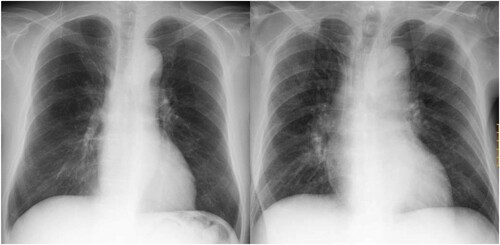
Table 1. Analysis of FLT3-ITD VAF in bone marrow and peripheral blood.
Since the differentiation of leukemic blasts and DS were strongly suspected, we retrospectively analyzed the genetic status of samples of peripheral blood and bone marrow. We obtained genomic DNA from blood smears and bone marrow smears (DNA IQTM System, Promega) and analyzed FLT3-ITD VAF as previously described () [Citation12]. At the time of relapse, bone marrow examination showed hypercellular marrow and the leukemic blast percentage was 85.0%. FLT3-ITD VAF was 95.6%. At day 20, the blast percentage was reduced to 15.0% and normal hematopoiesis of erythrocytes and myelomonocytes was observed with FLT3-ITD VAF of 93.2%. At day 77, bone marrow was normocellular: the blast percentage was 1.5% and FLT3-ITD VAF had decreased slightly to 77.5%. At day 167, FLT3-ITD VAFs in bone marrow and peripheral blood were more than 90%, regardless of the percentage of leukemic blasts. At day 293, blasts were not observed in peripheral blood, but FLT3-ITD VAF was 88.2%. This means that leukemic blasts differentiated upon treatment with gilteritinib.
Discussion
In our case, FLT3-ITD mutation was negative at disease onset but became positive at relapse. It is well established that FLT3 mutation positivity at relapse is sometimes different from findings at diagnosis [Citation13–15]. Examination of FLT3 mutation is strongly recommended not only at the time of disease onset but also at the time of relapse since FLT3 inhibitors are available for patients with R/R-AML.
Recent studies have shown that there are two types of responses to treatment with FLT3 inhibitors in patients with FLT3-mutated R/R-AML. One type is differentiation of leukemic blasts upon treatment with a FLT3 inhibitor and FLT3-ITD VAF being stable despite a decrease in the blast percentage. The other type is a cytotoxic response without differentiation in which a decrease of leukemic blasts is accompanied by reduction of both cellularity and FLT3-ITD VAF. These two types of responses are observed in both patients treated with gilteritinib and patients treated with quizartinib [Citation4,Citation16–18]. In addition, some of the patients responding to a FLT3 inhibitor with differentiation develop DS [Citation4,Citation17].
Compared with cases in the previous studies, our case showed a unique course. We would like to focus on two points. The first point is that we observed cytoreduction with a decrease of blasts in both bone marrow and peripheral blood at 2–3 months after the initiation of gilteritinib treatment. The second point is that DS was observed 6 months after the initiation of gilteritinib treatment.
As shown in , FLT3-ITD VAF in bone marrow was sustained at more than 90% throughout the clinical course except at day 77, although multiple cell lineages, i.e. granulocyte lineage, erythrocyte lineage and monocyte lineage, were observed in bone marrow examinations. In addition, FLT3-ITD VAFs in peripheral blood with few blasts at day 169 and day 293 were 93.9% and 88.2%, respectively. Considering the substantial percentage of each cell lineage in relation to FLT3-ITD VAF, leukemic blasts differentiated upon treatment with gilteritinib. At day 77, when the patient had achieved CRi, bone marrow was slightly hypocellular and FLT3-ITD VAF in bone marrow had declined to 77.5%. This indicates that gilteritinib not only induced differentiation of leukemic blasts but also transiently suppressed proliferation of FLT3-ITD-positive cells. It is well known that subclonal alterations are usually detected in AML cells from a single patient [Citation19]. Although we do not have evidence of clonal architecture of AML cells in our patient, we speculate the following possible scenario. During treatment with gilteritinib, there were two different types of FLT3-ITD-positive blasts. The first type of blasts was killed by gilteritinib. Therefore, cellularity of bone marrow was reduced until day 77. The second type of blasts, which might have existed before gilteritinib treatment or evolved from an ancestral clone during gilteritinib treatment, differentiated upon gilteritinib treatment but continued to proliferate. Therefore, peripheral blood at day 169 showed myelomonocytosis with DS, while leukemic blasts were proliferating in bone marrow.
DS was initially characterized in patients with acute promyelocytic leukemia who had been treated with all-trans retinoic acid and DS also occurred in patients who received treatment with arsenic trioxide [Citation11]. In patients treated with gilteritinib, DS was observed as early as 2 days and up to 75 days after gilteritinib initiation [Citation5]. It should be noted that DS was observed in our case 6 months after the initiation of gilteritinib treatment. Since both increases of granulocytes and monocytes in peripheral blood and proliferation of leukemia blasts in bone marrow were thought to be the main cause of DS, we conducted treatment to reduce the leukemia burden. Although chemotherapy during treatment with gilteritinib was not permitted in either the phase 1/2 CHRYSALIS study or the phase 3 ADMIRAL study, short-term chemotherapy effectively reduced the leukemia burden in our case. The resumption of gilteritinib treatment was effective for inducing differentiation of leukemic cells, while persistent leukemic blasts were observed in bone marrow. Although accumulation of evidence is necessary, sequential treatment with gilteritinib and chemotherapy might be effective in certain cases for management of AML with FLT3 mutation. Ongoing clinical trials may show the clinical benefit of sequential treatment with gilteritinib and chemotherapy [Citation20].
Statement of ethics
The authors have obtained written and signed consent to publish the case report from the legal guardian.
Conflict of interest
TK received honoraria from Astellas Pharma. The other authors declare no competing interests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Daver N, Schlenk RF, Russell NH, et al. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312.
- Perl AE, Altman JK, Cortes J, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol. 2017;18:1061–1075.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381:1728–1740.
- McMahon CM, Canaani J, Rea B, et al. Gilteritinib induces differentiation in relapsed and refractory FLT3-mutated acute myeloid leukemia. Blood Adv. 2019;3:1581–1585.
- DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood 2020; 135: 85–96.
- Sakaguchi M, Nakajima N, Yamaguchi H, et al. The sensitivity of the FLT3-ITD detection method is an important consideration when diagnosing acute myeloid leukemia. Leuk Res Rep. 2020;13:100198.
- Yamada K, Furusawa S, Saito K, et al. Concurrent use of granulocyte colony-stimulating factor with low-dose cytosine arabinoside and aclarubicin for previously treated acute myelogenous leukemia: a pilot study. Leukemia. 1995;9:10–14.
- Huls G, Chitu DA, Havelange V, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133:1457–1464.
- Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19:3244–3254.
- Tilly H, Castaigne S, Bordessoule D, et al. Low-dose cytosine arabinoside treatment for acute nonlymphocytic leukemia in elderly patients. Cancer. 1985;55:1633–1636.
- Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3:e2011059.
- Sakaguchi M, Yamaguchi H, Najima Y, et al. Prognostic impact of low allelic ratio FLT3-ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018;2:2744–2754.
- Kottaridis PD, Gale RE, Langabeer SE, et al. Studies of FLT3 mutations in paired presentation and relapse samples from patients with acute myeloid leukemia: implications for the role of FLT3 mutations in leukemogenesis, minimal residual disease detection, and possible therapy with FLT3 inhibitors. Blood. 2002;100:2393–2398.
- Shih LY, Huang CF, Wu JH, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392.
- Wakita S, Yamaguchi H, Omori I, et al. Mutations of the epigenetics-modifying gene (DNMT3a, TET2, IDH1/2) at diagnosis may induce FLT3-ITD at relapse in de novo acute myeloid leukemia. Leukemia. 2013;27:1044–1052.
- Yun HD, Nathan S, Larson M, et al. Erythroid differentiation of myeloblast induced by gilteritinib in relapsed. FLT3-ITD–positive acute myeloid leukemia. Blood Adv. 2019;3:3709–3712.
- Sexauer A, Perl A, Yang X, et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120:4205–4214.
- Nybakken GE, Canaani J, Roy D, et al. Quizartinib elicits differential responses that correlate with karyotype and genotype of the leukemic clone. Leukemia. 2016;30:1422–1425.
- Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510.
- Levis M, Shi W, Chang K, et al. FLT3 inhibitors added to induction therapy induce deeper remissions. Blood. 2020;135:75–78.
