ABSTRACT
Objectives
To evaluate hypomethylating agent (HMA) persistence in patients with myelodysplastic syndromes (MDS), and examine its association with healthcare resource utilization (HRU) and progression to acute myeloid leukemia (AML).
Methods
A total of 2,400 adults diagnosed with MDS initiating HMAs were included from IBM MarketScan databases during 1/1/2011–3/31/2018. The index date was HMA initiation following MDS diagnosis. Patients were classified according to their persistence status by the end of a fixed ‘landmark period’ of 4 months post-index.
Results
Median persistence to HMAs was 5.6 months (95% CI: 5.2, 6.1); HMA non-persistence increased with time. Non-persistent patients had a significantly higher non-HMA-related HRU burden than persistent patients [adjusted incidence rate ratios, outpatient visits: 1.12 (95% CI: 1.10, 1.14); inpatient visits: 1.48 (95% CI: 1.30, 1.69); emergency department visits 1.30 (95% CI: 1.12, 1.50); all p-values < 0.001]. All-cause and HMA-related outpatient visits were lower among non-persistent patients, likely because of fewer HMA administration-related visits. The incidence rate of AML was numerically, although not significantly, higher in non-persistent patients, when starting follow-up at the end of the landmark period. When follow-up began at the index date, non-persistent patients had a significantly higher rate of AML [adjusted hazard ratio, 1.88 (95% CI: 1.53, 2.32); p-value < 0.001].
Conclusions
HMA non-persistence, which increased over time, was associated with significantly higher non-HMA-related HRU, and numerically higher AML progression in MDS patients initiating HMAs. Future studies should evaluate predictors of HMA non-persistence in this patient population.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of myeloid disorders characterized by ineffective blood cell production, morbidities due to cytopenias, and a high risk of progression to acute myeloid leukemia (AML) [Citation1,Citation2]. In the US, the annual incidence rate of MDS was approximately 4.3 per 100,000 individuals during 2013–2017 [Citation3], with higher rates among older males [Citation1,Citation3,Citation4].
The only curative option for MDS is allogeneic hematopoietic stem cell transplant (HSCT) [Citation4]. However, due to the high risk of toxicity associated with HSCT, alternative therapies, such as hypomethylating agents (HMAs) (i.e. decitabine, azacitidine) are often considered [Citation1,Citation5]. Currently, decitabine is approved for use through intravenous (IV) administration [Citation6], and azacitidine through IV or subcutaneous (SC) administration [Citation7]. In clinical trials, HMAs have been associated with prolonged survival, hematological improvements, and delayed progression to AML compared to conventional care regimens [Citation8–11].
A key determinant of the success of HMA therapy is persistence to the recommended course of treatment [Citation12,Citation9], defined as a minimum of 4–6 cycles [Citation7,Citation6]. Nonetheless, evidence suggests that at least half of patients discontinue HMA therapy before completing the recommended course [Citation13,Citation14]. Reasons for this high prevalence of discontinuation are not well understood. However, one contributing factor may be the significant healthcare resource utilization (HRU) burden associated with IV and SC administration of HMAs across multiple cycles [Citation12,Citation15], each requiring in-person visits to healthcare settings for the recommended 3–7 days of drug administration [Citation6,Citation7].
The HRU burden associated with HMAs is likely further magnified among MDS patients who are not persistent to treatment, given that non-persistence is a known risk factor for worse health outcomes and increased economic burden [Citation12,Citation16,Citation17]. Indeed, two retrospective cohort studies reported a substantial HRU burden among MDS patients after 1st-line HMA failure [Citation15,Citation16]. However, to date, no studies have compared HRU outcomes among HMA persistent versus non-persistent patients. Additionally, although the HMA use has been shown to delay progression to AML [Citation8,Citation10,Citation11,Citation17], it is not clear if being persistent on HMAs is associated with delayed AML progression. Thus, there is a need to understand HMA persistence among patients with MDS, and examine its associations with HRU and AML progression. Accordingly, we conducted a study to evaluate HMA persistence, and HMA-related costs and HRU among patients with MDS initiating HMAs, and further examine the association of HMA persistence with HRU and AML progression.
Materials and methods
Study design
We conducted a retrospective cohort study using data from the IBM MarketScan Commercial Claims and Encounters database and the Medicare Supplemental and Coordination of Benefits database from 1/1/2011–3/31/2018. The Commercial database contains medical and drug data on several million individuals covered by employer-sponsored private health insurance, including employees and their spouses and dependents. The Medicare Supplemental database focuses on individuals aged ≥65 years with Medicare supplemental insurance paid by employers plus employer-paid commercial plans.
shows the study design schematic for this study. The index date was defined as the first HMA claim following a diagnosis of MDS. A 2-month washout period was implemented before the index date during which patients could not have used HMA therapy. The baseline period was the 6-month period before the index date. Given that both the exposure (HMA persistence) and outcomes (HRU and AML progression) were defined during the observation period, landmark analysis was used for evaluating the association of HMA persistence with HRU and AML progression. A fixed time period of 4 months after the index date was chosen as the landmark period. Patients who were in the database at the end of the landmark period, and still at risk of developing the outcome were classified according to their persistence status by that point. A 4-month landmark period was chosen because HMA treatment is recommended for at least 4 months (a minimum of 4 cycles is recommended, with each cycle typically being 4 weeks long), although more treatment cycles may be needed to achieve partial or complete response [Citation6,Citation7].
Figure 1. Study design scheme. AML: acute myeloid leukemia; HMA: hypomethylating agent; HRU: healthcare resource utilization; MDS: myelodysplastic syndromes.
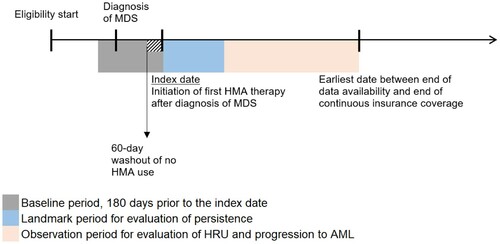
The observation period was defined differently for each outcome evaluated. For costs, the observation period spanned from the index date till the end of continuous eligibility, the end of data availability, the beginning of a ≥60-day gap in index HMA administration, or the day before a switch between azacitidine and decitabine, whichever occurred first. For comparing HRU across categories of HMA persistence, a landmark analysis was conducted, in which the observation period spanned from the day after the end of the 4-month landmark period till the end of data availability or the end of continuous insurance coverage, whichever occurred first. Only the first 6 months of the observation period were evaluated to make HRU outcomes for persistent and non-persistent patients more comparable, as follow-up duration was substantially shorter in the latter group; 6 months was chosen as it was the median follow-up duration in the non-persistent cohort. For comparing progression to AML across categories of HMA persistence, a landmark analysis was conducted, in which the observation period spanned from the day after the end of the 4-month landmark period till the end of data availability, the end of continuous insurance coverage, the first AML diagnosis, or the first HSCT procedure, whichever occurred first.
Study population
Patients who met the following eligibility criteria were included: confirmed diagnosis of MDS, defined as ≥2 International Classification of Diseases (ICD), 9th or 10th Revision, Clinical Modification (ICD-9-CM or ICD-10-CM) diagnosis codes for MDS (the first of these was required to be before September 30, 2017 to allow all patients the possibility of having an observation period of at least 6 months); ≥1 claim for HMA therapy following the first MDS diagnosis (before September 30, 2017); no claims for HMAs within 60 days before the index date; ≥6 months of continuous enrollment before the index date; ≥18 years of age as of the first MDS diagnosis; and no diagnosis of AML at or before the index date. The list of codes used to identify MDS are in Supplemental Table 1, those used to identify AML are in Supplemental Table 2, and those used to identify HMAs are in Supplemental Table 3.
For evaluating HRU, patients whose data availability or continuous insurance coverage ended before or at the end of the landmark period were excluded. For evaluating AML progression, patients who had an AML diagnosis or HSCT procedure or whose data availability or continuous insurance coverage ended before or at the end of the landmark period were excluded.
Study outcomes
Persistence was defined as the duration of HMA treatment (i.e. time from treatment initiation till discontinuation), where discontinuation was defined as the beginning of a gap of ≥60 days between claims for a particular HMA therapy. Patients who had not discontinued by the end of data availability or continuous insurance coverage or had switched were censored. Patients who received the initial HMA with <60 days of gap during the landmark period were defined as ‘persistent.’ Those with a ≥60-day gap in index HMA administration during the landmark period were defined as ‘non-persistent.’ Patients who initiated a second HMA during the landmark period prior to discontinuation (i.e. switched between azacitidine and decitabine) were defined as ‘switched.’
HMA-related healthcare costs were assessed on dates with any claim associated with HMA treatment. Medical costs comprised HMA IV or SC administration procedure costs and costs associated with other services provided on the day of the HMA treatment visit. Pharmacy costs comprised HMA drug costs and other drug costs. Costs were evaluated only for the year 2018, which was the most recent year of follow-up in the data.
All-cause, HMA-related, and non-HMA-related HRU during the first 6 months of the observation period were evaluated and reported by setting, including outpatient (OP) visits, inpatient (IP) visits, and emergency department (ED) visits. All-cause HRU was defined as all claims for HRU visits. HMA-related HRU was defined as all HRU on dates with any claim associated with HMA treatment. Non-HMA-related HRU was defined as all HRU on dates without any claim associated with HMA treatment. Progression to AML from MDS was assessed as having ≥1 claim(s) for AML over the observation period.
Statistical analysis
Patient characteristics, including age, sex, insurance plan type, Charlson-Quan comorbidity index (CCI) score and component conditions, therapies such as HSCT, HMAs, blood transfusions, and hematopoietic growth factors, and all-cause HRU were summarized at the index date, during the baseline period, or during the landmark period. The list of codes used to identify blood transfusions and hematopoietic growth factors are in Supplemental Table 4. Characteristics were summarized for the overall cohort, and stratified by categories of HMA persistence.
A categorical persistence variable was reported as the number and proportion of patients who were HMA persistent, HMA non-persistent, or had switched by the end of different landmark periods. Persistence status was evaluated during multiple landmark periods to understand how HMA persistence changes over time. A continuous persistence variable was summarized using means, standard errors (SE), and medians, computed using Kaplan-Meier methodology.
Per patient per month (PPPM) costs were calculated as the sum of all costs incurred by all patients during the observation period in 2018, divided by the sum of the lengths (in months) of the observation periods for all patients in 2018.
All-cause, HMA-related, and non-HMA-related HRU were calculated PPPM as the sum of all visits within that category incurred by all patients during the first 6 months of the observation period, divided by the sum of the lengths (in months) of the observation periods for all patients up to the first 6 months. HRU was summarized for the overall cohort, and stratified by categories of HMA persistence. Poisson regression models were used to report unadjusted and multivariable-adjusted incidence rate ratios (IRR) with 95% confidence intervals (CIs) comparing HRU rates across categories of HMA persistence. Multivariable models adjusted for age at landmark-end, sex, and region of residence, as well as CCI, receiving transfusions and growth factors, and number of all-cause visits of the type of the dependent variable during the baseline period.
Frequencies and proportions of patients with MDS who had ≥1 claim(s) for AML were summarized over the observation period for the overall cohort and by categories of HMA persistence. Median (95% CI) time to AML progression was reported using Kaplan-Meier methodology. Poisson regression models were used to report IRRs (95% CIs) for AML during the observation period. Cox proportional hazards regression models were used to report unadjusted and multivariable-adjusted hazard ratios [HR (95% CIs)] comparing the hazard of AML progression across categories of HMA persistence. Multivariable models adjusted for age at landmark-end, sex, and region of residence, as well as CCI, receiving transfusions, and receiving growth factors during the baseline period. In the main landmark analysis, follow-up started at the end of the 4-month landmark period. Additionally, an exploratory analysis was conducted in which follow-up started at the index date to evaluate how results might change if all AML cases occurring after HMA initiation were included in the analysis. All analyses were performed using SAS release 9.4 or newer version (SAS Institute, Cary, NC)
Results
A total of 41,338 patients with a confirmed diagnosis of MDS were identified. After applying the eligibility criteria, 2,400 patients were included in the HMA study cohort.
HMA persistence
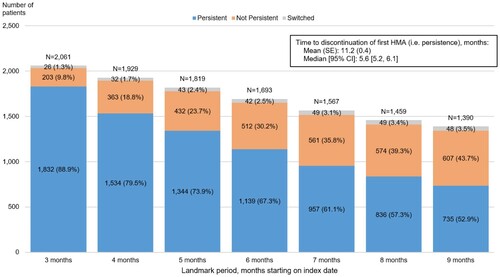
After HMA initiation, the median time to treatment discontinuation was 5.6 months (95% CI: 5.2, 6.1) (). When defining persistence based on whether or not a patient had a gap of ≥60 days in treatment before the end of the landmark period, HMA non-persistence increased over time. For instance, while 9.8% of patients were not persistent to HMAs during the 3-month landmark period, this figure almost doubled to 18.8% during the 4-month landmark period.
HMA-related costs
Mean HMA-related healthcare costs were $7,707 PPPM in 2018. Of these, $6,200 PPPM were medical service costs related to HMA administration procedures, which could be further broken down into costs associated with the HMA injection ($5,112 PPPM), and those associated with IV/SC procedures for administering it ($1,087 PPPM). Pharmacy costs were $308 PPPM in 2018, and all of these were for other, non-HMA drugs in pharmacy claims on HMA days, which included anti-infective agents, central nervous system agents, gastrointestinal drugs, cardiovascular agents, and other drugs.
HRU
After excluding 471 patients whose data availability or continuous insurance coverage ended before or at the end of the landmark period, 1,929 patients remained for evaluating HRU. Of these, 1,534 remained persistent, 363 became non-persistent, and 32 switched between decitabine and azacitidine by the end of the 4-month landmark period. Given the small number of patients who switched between HMA treatments, results for the rest of the analyses focus on persistent and non-persistent patients only.
shows baseline characteristics of the patients. Mean age at the index date was 71.3 and 70.1 years in the persistent and non-persistent cohorts, respectively, with 36.0% and 33.3% of patients being female. Median time from index date to the end of the observation period was longer in the persistent (15.0 months) than the non-persistent cohort (9.8 months). Mean CCI scores were similar in persistent and non-persistent patients. As supportive care, 40.4% and 42.1% of persistent and non-persistent patients received blood transfusions, while 38.9% and 38.6% received hematopoietic growth factors. During the landmark period, mean CCI scores were 1.8 and 2.4 in the persistent and non-persistent cohorts, respectively (Supplemental Table 5). As supportive care, 55.7% and 60.3% of persistent and non-persistent patients received blood transfusions, while 52.0% and 53.7% received hematopoietic growth factors.
Table 1. Patient characteristics during the baseline period stratified by persistence to HMAs at 4 months.
During the first 6 months of the observation period, in the persistent and non-persistent cohorts respectively, there were 9.87 and 8.29 all-cause OP visits, 3.09 and 0.28 HMA-related OP visits, and 6.78 and 8.00 non-HMA-related OP visits PPPM (). In both the persistent and non-persistent cohorts, there were fewer all-cause IP visits (0.12 and 0.20 PPPM, respectively) and ED visits (0.13 ad 0.16 PPPM, respectively); most of these were non-HMA-related. The number of all-cause IP days across all IP visits PPPM were 1.56 in the persistent cohort, and 2.69 in the non-persistent cohort, again with most of these being non-HMA-related.
Table 2. Descriptive statistics on healthcare resource utilization during the first 6 months of the observation period, stratified by persistence at 4 months
In both unadjusted and adjusted analyses, non-persistent patients had a significantly higher non-HMA related HRU burden relative to persistent patients (). For instance, multivariable-adjusted IRRs comparing non-persistent to persistent patients were 1.48 (95% CI: 1.30, 1.69) for non-HMA-related IP visits and 1.41 (95% CI: 1.36, 1.46) for non-HMA-related IP days, with all p-values being <0.001. The same was true for all-cause IP visits, IP days, and ED visits, which were significantly higher among non-persistent than persistent patients. All-cause and HMA-related OP visits were lower among non-persistent than persistent patients which was expected given that persistent patients likely visit OP settings more frequently for HMA administrations. Few patients had HMA-related IP or ED visits in this cohort, and results for these could not be reported due to models not converging or values being undefined.
Table 3. Unadjusted and adjusted analyses on healthcare resource utilization during the first 6 months of observation period.
AML progression
After additionally excluding patients who developed AML or received an HSCT procedure during the landmark period, 1,663 patients were included in the analysis for AML progression. Mean age at index date among persistent and non-persistent patients was 71.8 and 73.9 years, respectively (Supplemental Table 6). During the baseline period, mean CCI scores were 2.0 and 2.2 among persistent and non-persistent patients, respectively. Similar proportions of persistent and non-persistent patients received blood transfusions (40.4% vs 40.9%, respectively) and hematopoietic growth factors (40.3% vs 42.6%, respectively) during the baseline period. During the landmark period, persistent and non-persistent patients diverged slightly more in terms of their mean CCI scores (1.7 vs 2.1, respectively), receiving blood transfusions (54.4% vs 56.6%, respectively), and receiving hematopoietic growth factors (53.3% vs 57.4%, respectively) (Supplemental Table 7).
Over the observation period, 21.8% of persistent and 16.1% of non-persistent patients developed AML (). Mean time to AML progression was 38.5 months in the persistent cohort and 22.0 months in the non-persistent cohort. The incidence rate of AML per 100 patient months was numerically higher in non-persistent patients [1.73 (95% CI: 1.27, 2.37)] relative to persistent patients [1.53 (95% CI: 1.36, 1.71)], when starting follow-up at the end of the 4-month landmark period. However, the multivariable-adjusted hazard ratio did not reach statistical significance [1.09 (95% CI: 0.78, 1.53; p = 0.612)]. In an exploratory analysis where follow-up began at the index date, non-persistent patients had a significantly higher rate of AML, with the multivariable-adjusted HR being 1.88 (95% CI: 1.53, 2.32; p < 0.001).
Table 4. Unadjusted and adjusted analyses on progression to AML.
Discussion
In this study among patients with MDS initiating HMA treatment in the US, persistence to HMAs decreased over time. Overall, mean HMA-related healthcare costs were $7,707 PPPM, of which ∼80% comprised HMA administration-related medical costs. All-cause IP visits, IP days, and ED visits, and non-HMA-related visits of all types were significantly higher in non-persistent relative to persistent patients, suggesting higher HRU burden in non-persistent patients. HMA-related OP visits were higher in the persistent group, likely due to more frequent OP visits for HMA IV/SC administrations. Finally, we found indications of a higher rate of progression to AML in non-persistent relative to persistent patients, although the results were not statistically significant.
Prior studies have shown HMA non-persistence to be highly prevalent in real-world settings [Citation13,Citation14]. For instance, in one study, ∼50% of MDS patients showed early HMA discontinuation defined as the completion of <5 HMA cycles of a given HMA [Citation13]. In another study, 32% of patients who initiated HMA therapy discontinued after the first cycle, and 69% discontinued before reaching 6 cycles [Citation14]. This is line with our finding of decreasing HMA persistence over time. While we found a lower proportion of patients discontinuing treatment relative to these studies, this is likely due to how persistence was defined in our study. To be classified as non-persistent, patients had to have a ≥60-day gap in treatment before the end of the landmark period. While this was done to avoid bias when comparing outcomes across persistent and non-persistent groups in the landmark analysis, it could have led to an underestimation of non-persistence by the end of specific landmark periods. For instance, with a 4-month landmark period, patients would need to discontinue treatment by the 2-month mark at the latest to have ≥60 days of no treatment and be classified as non-persistent. Per this definition, patients discontinuing treatment at the 3- or 4-month marks wouldn’t get classified as non-persistent. Such patients are likely captured as non-persistent in the other studies described above, making their estimates of the proportions of non-persistent patients higher than those found in this study. Nevertheless, when evaluating persistence over the entire study period, we found that ∼50% of patients discontinued treatment by 5.6 months (i.e. median time to discontinuation), which is in line with the previous research summarized above.
Few studies have explored factors contributing to HMA non-persistence. However, evidence suggests that a vast majority of oncology patients prefer oral therapies over IV/SC administered therapies, citing greater convenience, avoidance of venipunctures, and a greater sense of control as primary motivating factors [Citation18–20]. A recent survey of MDS patients found that the desire to be at home rather than in a hospital was a primary factor influencing their treatment decisions [Citation21]. Therefore, the high HRU burden and inconvenience associated with IV/SC administration may be a contributing factor to HMA non-persistence. However, further research is required to confirm this.
We found persistent patients to have significantly higher HMA-related OP visits relative to non-persistent patients in this study. This is expected, given persistent patients are visiting OP settings more frequently to receive their HMA administrations. Cogle et al. [Citation15] found MDS patients on HMA therapy to have on average 42.7 MDS-related office visits during a one-year period (or ∼3.56 visits per month), similar to our study in which we found persistent patients to have 3.11 HMA-related OP visits PPPM.
We also found significantly higher all-cause and non-HMA-related HRU burden among non-persistent relative to persistent patients. To our knowledge, no other comparisons of HRU outcomes in HMA persistent versus non-persistent patients have been conducted. However, a small number of studies have assessed HRU in patients after HMA therapy failure and found the burden to be substantial [Citation15,Citation16]. For instance, in one study [Citation16], MDS patients had 25.9 all-cause physician office visits on average during the 6 months after first-line HMA failure (i.e. ∼4.3 visits per month), with 32.6% and 19.7% of patients having at least 1 all-cause IP and ED visit, respectively. The corresponding numbers in our study were slightly higher, with 8.29 all-cause OP visits PPPM among non-persistent patients during the 6 months after the landmark period, and 46.6% and 24.0% of non-persistent patients having at least 1 all-cause IP and ED visit, respectively. In another study in which patients were assessed before and after first-line failure, a high HRU burden was also observed post-HMA failure [Citation15]. However, given that the pre- and post-HMA failure periods were not of the same length, the HRU estimates are not readily comparable. Our study adds to the existing literature by demonstrating high HRU burden among MDS patients who are not persistent on HMAs relative to those who are.
In the main landmark analysis, rates of AML were numerically but not significantly higher in non-persistent versus persistent patients. However, in an exploratory analysis where follow-up began at the index date, progression to AML was significantly higher among non-persistent relative to persistent patients. Unlike the landmark analysis, this exploratory analysis captured information from all patients who developed AML after HMA initiation. This indicates that a substantial number of AML cases occurred during the landmark period, and these events were more highly concentrated among non-persistent patients. The landmark analysis was restricted to patients who were still alive and had not developed AML by the end of the 4-month landmark period, thereby missing cases of AML that developed soon after HMA initiation by design. Relative to persistent patients, non-persistent patients were older, had higher mean CCI scores, and received more supportive therapies during the landmark period. It is possible that these patients were sicker or had more severe disease, and hence were more prone to developing AML early, which would not have been captured in the landmark analysis. Thus, the exploratory analysis provides a more complete picture of the clinical experience of this patient population. However, this analysis could be subject to reverse causation, as some AML cases during the landmark period could have preceded non-persistence. Thus, landmark analysis is methodologically more robust to analyze the association between persistence and AML progression. Nevertheless, in this instance, this analysis was unable to include a considerable number of AML events, and the results from it may not be representative of all MDS patients who initiate HMA treatment. Previous studies have reported a negative impact of HMA therapy failure on other clinical outcomes including overall survival and progression-free survival [Citation12,Citation22]. However, to our knowledge, no studies have compared time to AML progression among HMA persistent and non-persistent patients. Thus, our study addresses a key gap in the current literature.
This study is subject to limitations inherent to retrospective claims analyses. Claims data may have coding inaccuracies or omissions, although these are likely to affect persistent and non-persistent cohorts similarly. Additionally, claims codes are used primarily for billing purposes, and thus coding patterns may cater more to reimbursement than clinical purposes, leading to potential misclassification. Claims codes also lack clinical detail, making it difficult to characterize patients by disease severity or other clinical factors. In particular, patients with all types of MDS were included in this study, and hence they likely represent a heterogeneous patient population in terms of disease prognosis. Given that this claims database doesn’t have clinical information such as International Prognostic Scoring System (IPSS) and Revised IPSS (IPSS-R) scores, the analyses couldn’t be stratified by indicators of prognosis. Additionally, as mentioned previously, landmark analysis has its own limitations, which must be considered when interpreting the results. Lastly, the present study assessed outcomes in patients enrolled in commercial and managed care plans and may, therefore, not be generalizable to patients on other insurance plans (e.g. Medicaid) or outside of the US.
Conclusions
HMA non-persistence, which increased over time, was associated with significantly higher non-HMA-related HRU burden in patients with MDS initiating HMAs. There was also evidence of a higher rate of progression to AML among non-persistent relative to persistent patients, although these results weren’t statistically significant in the landmark analysis. Future studies should evaluate predictors of HMA non-persistence in this patient population, to better contextualize the observed increase in HMA non-persistence over time, and its potential clinical and economic impact.
Supplemental Material
Download MS Word (35.8 KB)Acknowledgements
We thank Tiffani Shovlin (Taiho Oncology, Inc.) for her inputs to the analysis, and Mona Lisa Chanda (Analysis Group, Inc.) for providing medical writing assistance.
Disclosure statement
Wendy Y Cheng, Ambika Satija, Hoi Ching Cheung, François Laliberté, and Patrick Lefebvre are employees of Analysis Group, Inc., which received funding from Taiho Oncology, Inc. to conduct the research study and develop this manuscript. Kala Hill and Tim Wert are employees of Taiho Oncology, Inc.
Data availability statement
The data that support the findings of this study are available from IBM MarketScan. Restrictions apply to the availability of these data, which were used under license for this study.
Ethics approval: This study used de-identified MarketScan data, which comply with the Health Insurance Portability and Accountability Act. Thus, ethical approval was not necessary for this study.
Consent to participate: Given this study used de-identified HIPPA-compliant MarketScan data, formal consent was not obtained.
Additional information
Funding
References
- Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(1):60–87. doi:https://doi.org/10.6004/jnccn.2017.0007.
- Ades L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383(9936):2239–2252. doi:https://doi.org/10.1016/S0140-6736(13)61901-7.
- National Cancer Institute Surveillance E, and End Results (SEER) Program. (2017). SEER Cancer Statistics Review 1975-2017.
- Kobbe G, Schroeder T, Haas R, et al. The current and future role of stem cells in myelodysplastic syndrome therapies. Expert Rev Hematol. 2018;11(5):411–422. doi:https://doi.org/10.1080/17474086.2018.1452611.
- National Comprehensive Cancer Network. (2020). NCCN Clinical practice guidelines in oncology - Myelodysplastic syndromes version 2.2020 [cited 2020 May 12]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf.
- US Food and Drug Administration. (2020). Prescribing information - DACOGEN® (decitabine) [cited 2020 May 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021790s023lbl.pdf.
- US Food and Drug Administration. (2020). Prescribing information - VIDAZA (azacitidine) [cited 2020 May 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050794s032lbl.pdf.
- Scott LJ. Azacitidine: a review in myelodysplastic syndromes and acute myeloid leukaemia. Drugs. 2016;76(8):889–900. doi:https://doi.org/10.1007/s40265-016-0585-0.
- Killick SB, Carter C, Culligan D, et al. Guidelines for the diagnosis and management of adult myelodysplastic syndromes. Br J Haematol. 2014;164(4):503–525. doi:https://doi.org/10.1111/bjh.12694.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440. doi:https://doi.org/10.1200/jco.2002.04.117.
- Kantarjian H, Issa J-PJ, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer. 2006;106(8):1794–1803. doi:https://doi.org/10.1002/cncr.21792.
- Cabrero M, Jabbour E, Ravandi F, et al. Discontinuation of hypomethylating agent therapy in patients with myelodysplastic syndromes or acute myelogenous leukemia in complete remission or partial response: retrospective analysis of survival after long-term follow-up. Leuk Res. 2015;39(5):520–524. doi:https://doi.org/10.1016/j.leukres.2015.03.006.
- Mukherjee S, Cogle CR, Bentley TG, et al. Treatment patterns among patients with myelodysplastic syndromes: observations of 1st-line therapy, discontinuation and the need of additional therapies. Blood. 2014;124(21):2598. doi:https://doi.org/10.1182/blood.V124.21.2598.2598.
- Demakos EP, Silverman LR, Lawrence ME, et al. Incidence and treatment of myelodysplastic syndrome in the US: treatment approaches, optimization of care and the need for additional therapeutic agents. Blood. 2014;124(21):1287. doi:https://doi.org/10.1182/blood.V124.21.1287.1287.
- Cogle CR, Mukherjee S, Bentley TG, et al. Healthcare resource utilization and costs among patients with myelodysplastic syndrome who failed 1st-line therapy. Blood. 2014;124(21):2627.
- Cogle CR, Kurtin SE, Bentley TG, et al. The incidence and health care resource burden of the myelodysplastic syndromes in patients in whom first-line hypomethylating agents fail. Oncologist. 2017;22(4):379–385. doi:https://doi.org/10.1634/theoncologist.2016-0211.
- Cogle CR. Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep. 2015;10(3):272–281. doi:https://doi.org/10.1007/s11899-015-0269-y.
- Eek D, Krohe M, Mazar I, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence. 2016;10:1609–1621. doi:https://doi.org/10.2147/PPA.S106629.
- Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399. doi:https://doi.org/10.2147/PPA.S101821.
- O'Neill VJ, Twelves CJ. Oral cancer treatment: developments in chemotherapy and beyond. Br J Cancer. 2002;87(9):933–937. doi:https://doi.org/10.1038/sj.bjc.6600591.
- Booth A, Bell T, Halhol S, et al. Using social media to uncover treatment experiences and decisions in patients with acute myeloid leukemia or myelodysplastic syndrome who are ineligible for intensive chemotherapy: patient-centric qualitative data analysis. J Med Internet Res. 2019;21(11):e14285. doi:https://doi.org/10.2196/14285.
- Prebet T, Fenaux P, Vey N, et al. Predicting outcome of patients with myelodysplastic syndromes after failure of azacitidine: validation of the North American MDS consortium scoring system. Haematologica. 2016;101(10):e427–e428. doi:https://doi.org/10.3324/haematol.2016.150714.