ABSTRACT
Background
Acquired von Willebrand syndrome (AVWS) is a rare, frequently underdiagnosed and underestimated bleeding disorder. Careful personal and family history and late-onset mucocutaneous bleeding could help clarify the etiology of bleeding deficiency.
Case presentation
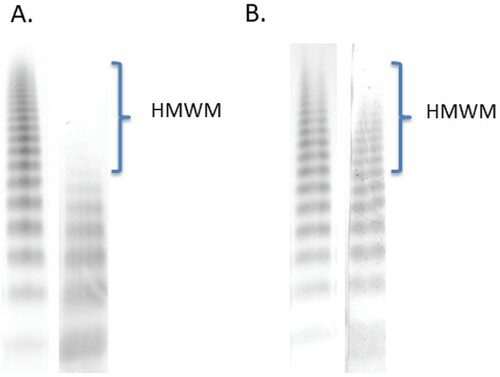
An 82-year-old male patient was admitted to our clinic with a severe nosebleed on 30.05.2018. Laboratory results revealed thrombocytosis, elevated white blood cell count and high LDH. Basic coagulation parameters were normal. He was referred to our clinic, where a bone marrow biopsy was taken. His personal and family history had no mention of bleeding disorders, nor was he on anticoagulant therapy. We detected elevated VWF antigen and decreased VWF ristocetin cofactor activity. Loss of high molecular weight multimers was detected by using agarose gel electrophoresis. These laboratory results were indicative of AVWS. Hydroxyurea treatment was initiated, leading to a gradual decrease in platelet count. The histological examination revealed essential thrombocytosist while mutation analysis was JAK2/CALR/MPL negative. However, due to severe nosebleeds, the patient was hospitalized and needed blood transfusion. A cardiological check-up revealed the progression of aortic valve stenosis. After, balloon-dilation a transcatheter aortic valve implantation was performed. As a result, VWF activity and activity to antigen ratio returned to normal as did multimeric structure. In July 2019, the follow-up examination showed that the patient was in a satisfactory condition, with normal hematological parameters, and no new nosebleed episode occurred
Conclusions
The patient complained of recurring nosebleeds, which stopped completely after the resolution of both underlying conditions successful cytoreductive treatment of triple-negative ET and transcatheteric aortic valve replacement.
Introduction
Acquired von Willebrand syndrome (AVWS) is a rare, frequently underdiagnosed, but potentially life-threatening bleeding disorder [Citation1]. Late-onset typically mucocutaneous bleeding and a negative personal and family history should always raise the possibility of AVWS, with regard to the fact that mild von Willebrand disease (VWD) can remain asymptomatic for decades [Citation2]. The majority of cases are related to haematological disorders (lympho-and myeloproliferative neoplasms), cardiovascular diseases (commonly aortic valve stenosis), solid cancers (Wilms tumor), autoimmune disorders (systemic lupus erythematosus), uremia and hypothyreosis [Citation2,Citation3]. The diagnosis is based on the determination of the level of von Willebrand factor (VWF:Ag) and its function by assays like VWF activity (VWF:Act), or ristocetin cofactor assay (VWF:RCo) and collagen binding capacity (VWF:CB). Our laboratory formerly used the traditional VWF ristocetin cofactor assay and still kept the abbraviation VWF:RCo when reporting the platelet binding properties of VWF. However, currently the Innovance VWFAc assay is in use (Siemens, Marburg, Germany) which is a VWF:GPIbM assay. Since this assay shows a very good correlation with the gold standard VWF:RCo test, the laboratory considered is acceptable to use the ‘old’ nomenclature when the activity of VWF was reported [Citation4,Citation5]. The decrease of high molecular weight multimers (HMWM) can be indicated by a reduced VWF:RCo/VWF:Ag ratio or VWF:CB/VWF:Ag ratio and it is confirmed by VWF multimeric analysis [Citation6,Citation7]. Hereby, we present a case with two possible causes of AVWS.
Case report
An 82-year-old patient was admitted to the Department of Otolaryngology and Head and Neck Surgery, with a severe nosebleed on 30 May 2018. His medical history included hypertension, type 2 diabetes mellitus and significant aortic valve stenosis (peak gradient 74 mm Hg, mean gradient 44 mm Hg, Vmax: 4.31 m/s, aortic area: 1.08 cm2) diagnosed in October 2017. Laboratory results revealed thrombocytosis (1537 G/L), elevated white blood cell count (11.67 G/L), high LDH (443 U/L) and a moderate CRP elevation (14 mg/L). Basic coagulation parameters were found normal. He was referred to our clinic on 1 June 2018 for haematological consultation. Considering the clinical signs and laboratory findings, a chronic myeloproliferative neoplasm and a secondary bleeding disorder were suspected. Bone marrow biopsy was taken and a sample for driver mutation (JAKV617F) was sent to the Department of Pathology and to the Department of Laboratory Medicine. His personal and family history was negative for a bleeding disorder, and no anticoagulant/antiplatelet therapy had been administered earlier. PFA-100 analysis indicated impaired platelet function, the closure times were prolonged with both cartridges (adrenalin/collagen, ADP/collagen). We detected elevated VWF antigen (VWF:Ag, 206%) and decreased VWF ristocetin cofactor activity (VWF:RCo, 61%), the VWF:RCo/VWF:Ag ratio was 0,32. Although the VWF collagen binding activity (VWF:CB, 91%) value is within the normal range, the VWF:CB/VWF:Ag ratio is low (0.44), and factor VIII coagulant activity (FVIII:C, 156%) were normal (). Loss of HMWM of VWF was detected by using agarose gel electrophoresis (). These laboratory results were indicative of AVWS. Hydroxyurea (HU) treatment (1000 mg/day) was urgently initiated to reduce platelet count and to resolve nosebleeding. Meanwhile, the histological examination of the trephine biopsy confirmed essential thrombocythemia (ET, increased number of pathological megakaryocytes, without significant reticulin fibrosis, not illustrated). The JAK2V617F/CALR/MPL mutation analysis gave negative results. Cytoreductive treatments led to the gradual normalization of the platelet count, the VWF:RCo level returned to normal (173%), but the VWF:RCo/VWF:Ag ratio (0.53) remained decreased and the nosebleeds went on. Through investigation of the gastrointestinal tract (gastroscopy, colonoscopy) revealed neither angiodysplasia nor any other source of bleeding. The patient’s condition required otolaryngological management several times between June 2018 and August 2018. On 14 August 2018, he was referred to our Department of Cardiology because of NYHA stage IV. heart failure (pro-BNP level 5483 ng/mL). Control echocardiography confirmed progressing severe calcifying aortic valve stenosis (peak gradient 101 mmHg, mean gradient 64 mmHg, Vmax: 5.08 m/s. aortic area: 0.7 cm2). The patient was refferred to an invasive cardiological examination. CT angiography of the head and neck region did not detect any morphological disturbances. Hereditary hemorrhagic teleangiectasia was excluded by gene sequencing of the endoglin gene. The heavy nosebleeds caused progressive anemia occurred and he received a total of six bags of red blood cells before cardiology admission. In October 2018, he underwent a coronary angiography, which showed a chronic right coronary occlusion with significant stenosis of the left anterior descending artery. Drug-eluting stent (3 × 14 mm) implantation was successfully performed and dual antiplatelet therapy (acetylsalicylic acid and clopidogrel) was initiated. Transcatheter aortic valve replacement (27 mm biological valve insertion) was successfully performed in December 2018. After his discharge from the Cardiology Unit, he had only one episode of nosebleeds. The VWF:Ag level (175%) and VWF:RCo/VWF:Ag ratio (0.75) and the multimeric structure of VWF normalized. In June 2019, he was in a good clinical condition, he did not report any nosebleeding and heart-related problems. He was administered HU 1000 mg/day.
Figure 1. Platelet count and acquired von Willebrand syndrome-related laboratory parameters before treatment, after cytoreductive therapy and after successful transcatheteric ortic valve replacement. The gray shaded area indicated the normal range.
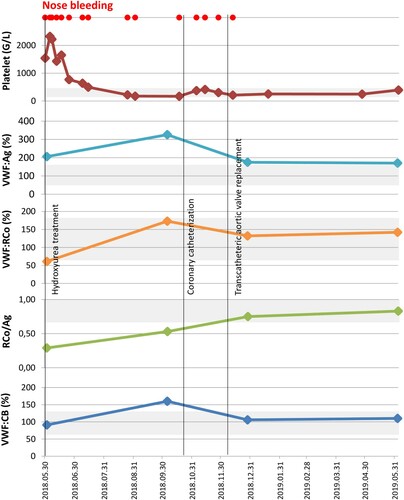
Figure 2. Loss of high molecular weight multimers (A, 1 June 2018) of von Willebrand factor (VWF) and normal multimeric structure (B, 28 December 2018) of VWF were detected by using 0.8% agarose gel electrophoresis. SDS–agarose gel electrophoresis was performed in two separate experiments according to the date of collecting blood sample from the patient, however, the same control plasma pool was used in both experiments. Lane 1, control plasma pool; Lane 2, patient’s plasma. HMWM are indicated in the figure.
Conclusions
AVWS is a rare bleeding disorder with similar clinical signs to the inherited form of VWD. Careful personal and family history taking could help identify the etiology of the bleeding deficiency. AVWS can be associated with a number of conditions and the management of AVWS should be directed at the underlying disorder [Citation8]. Extreme thrombocytosis (platelet count more than 1500 G/L) is frequently associated with AVWS. Based on the current treatment algorithm, urgent cytoreductive treatment is required [Citation9]. Clinically, ET and the prefibrotic/early stage of myelofibrosis can be very similar. Differential diagnosis is based on the results of histological examination of the bone marrow, which also helps explore treatment options and detect prognosis [Citation10]. In our patient the JAK2V617F, calreticulin and MPL mutations were negative, but the bone marrow biopsy confirmed the diagnosis of ET. HU treatment led to the gradual normalization of the thrombocyte count. The frequency of nosebleeding also decresased but severe episodes still occured. Beside the haematological disease, the patient also had significant aortic stenosis. Based on the literature data aortic valve stenosis is known to be associated with loss of HMWM, causing AVWS [Citation11]. In 2007, Schödel et al. described the nasopharyngeal analougue of Heyde syndrome [Citation12]. The patient complained of frequent recurring nosebleeds, which stopped completely after the resolution of both underlying conditions successful cytoreductive treatment of triple-negative ET and transcatheteric aortic valve replacement. Our case is a good example for the complexity of a disease and it underlines that in atypical cases several causative factors have to be considered. It also emphasizes the importance of cooperation between hematologist, cardiologist and laboratory scientist.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rottenstreich A, Kleinstern G, Krichevsky S, et al. Factors related to the development of acquired von Willebrand syndrome in patients with essential thrombocythemia and polycythemia vera. Eur J Intern Med. 2017;41:49–54.
- Tiede A, Rand JH, Budde U, et al. How I treat the acquired von Willebrand syndrome. Blood. 2011;117(25):6777–6785.
- Federici AB, Rand JH, Bucciarelli P, et al. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. 2000;84(2):345–349.
- Szederjesi A, Baronciani L, Budde U, Castaman G, Lawrie AS, Liu Y, et al. An international collaborative study to compare different von Willebrand factor glycoprotein Ib binding activity assays: the COMPASS-VWF study. J Thromb Haemost. doi:https://doi.org/10.1111/jth.14206. Online ahead of print.
- Bodó I, Eikenboom J, Montgomery R, et al. Platelet-dependent von Willebrand factor activity: nomenclature and methodology: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(7):1345–1350.
- Tiede A. Diagnosis and treatment of acquired von Willebrand syndrome. Thromb Res. 2012;130: S2–S6.
- Mital A. Acquired von Willebrand syndrome. Adv Clin Exp Med. 2016;25(6):1337–1344.
- Federici A, Budde U, Castaman G, et al. Current diagnostic and therapeutic approaches to patients with acquired von Willebrand syndrome: a 2013 update. Semin Thromb Hemost. 2013;39(2):191–201.
- Tefferi A, Vannucchi AM, Barbui T. Essential thrombocythemia treatment algorithm 2018. Blood Cancer J. 2018, 8(2). DOI https://doi.org/10.1038/s41408-017-0041-8
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405.
- Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349(4):343–349.
- Schödel J, Obergfell A, Maass AH. Severe aortic valve stenosis and nosebleed. Int J Cardiol. 2007;120(2):286.–287.
