ABSTRACT
Ataxia telangiectasia (A-T) is a rare childhood autosomal recessive neurodegenerative chromosomalin stability disorder. It is characterized by high risk of hematological malignancies with T-cell phenotype being the most common, which can present first before the diagnosis of A-T made. The chromosomalin stability in A-T increases the toxicity to radio-chemotherapeutic agents, creating the treatment modification challenges and the deviation from the optimal management protocols. In this case report, we present a 14-month-old boy diagnosed as T cell –ALL. Based on his early presentation, family history of childhood lymphoma, and high AFP, inherited predisposition was suspected, and genetic testing confirms A-T. This report represents the crucial part of clinical suspicion of A-T in similar cases as well as highlighting the importance of an early A-T diagnosis that prevents toxic death due to the extensive regimen of radio- chemotherapeutic agents. The report summarizes the toxicity and modification challenges during management with literature review for the chemotherapy modification experience in such cases.
Background
Ataxia telangiectasia (A-T) is a complex, multisystem, rare autosomal recessive disorder caused by mutations in the ATM (ataxia telangiectasia mutated) gene on chromosome 11q.26 [Citation1]. Patients usually present before the age of three years with progressive cerebellar ataxia; however, other neurological manifestation including ocular apraxia and dysarthria and oculocutaneous telangiectasia developed a few years later. Almost 50% of patients with A-T have variable degrees of immune deficiency and can present with recurrent Sino-pulmonary infection. In comparison to general population, patients with A-T have 100 folds that increase the risk of developing hematological malignancies, mainly aggressive Non-Hodgkin lymphoma, acute leukemia, and Hodgkin lymphoma. Among acute leukemia, T-cellis the commonest phenotype [Citation2]. Some patients may present with cancer before the diagnosis of A-T is made. Early diagnosis might be suspected if there is positive family history, early onset malignancy, and an unusual severe toxicity after chemotherapeutic treatment [Citation3]. Serum alpha-fetoprotein is a simple, rapid, and reliable screening test for A-T; it is proven to be elevated in more than 90% of A-T cases [Citation3]. Increasing toxicity in A-T patients with acute leukemia after radio- and chemotherapeutic treatment remains a subject of debate. [Citation2]
In this paper, we discuss the value of AFP in raising the suspicions of the disease, and the importance of the early diagnosis of A-T which can prevent the patient from toxic death as a result of radio-chemotherapy toxicity. After analyzing the modification and outcome in each chemotherapy phase, we found that despite the modification of the patient’s chemotherapy, he had an early and rapid neurological toxicity in his last chemotherapy phase.
We conclude that the optimal treatment of malignancy in A-T patients is not well defined due to the variation in phenotypes and clinical presentations; therefore, it is difficult to standardize the therapy. Appropriate modification of the therapy should be individualized and continuously reviewed at each stage without disregarding several factors as age at presentation, clinical condition, and the immune deficiency status associated with A-T. Among published cases, we found no evidence of a preferred strategy as each has its cons and pros. As we seek to elucidate the optimal treatment strategy in future, we are optimistic that reporting similar case presentations, diagnostic strategies, chemotherapy modifications, toxicity, and toxic death in consequence may be of greatbenefit
Case presentation
We are presenting 14 months old boy who was born to a healthy consanguine parent. He is a product of full-term, uncomplicated pregnancy and delivery. His eldest sister is healthy; however, his older brother died of sepsis at the age of 18 months shortly after being diagnosed with lymphoblastic lymphoma at our hospital.
Our patient was transferred to our PICU after an acute onset of shortness of breath and cyanosis as a case of mediastinal mass for diagnosis and management. Upon admission, he had a picture of superior vena cava syndrome and hepato-splenomegaly; however, there was no clinically palpable lymph node. CNS and testicular exam were unremarkable. The initial labs showed leukocytosis (>70 × 109/L), elevated LDH, elevated uric acid, mildly low immunoglobulin (IgG) level and high alpha-feto protein (). He was intubated on day 2 of admission and mechanically ventilated for 16 days. The initial Chest X-ray showed enlarged mediastinum with subtotal opacification in the right hemi-thorax along with bilateral pleural effusion ((a)). Chest CT revealed a mediastinal mass compressing on the lower part of trachea ((a)). The peripheral blood sample flow cytometry was consistent with T-cell Lymphoblastic Leukemia/ Lymphoma. There was 16% cell expressing CD1a, CD2, cyCD3(bright), CD4, CD5, CD7, CD8(partial/dim), CD45 & CD123; negative for smCD3, CD10, CD13, CD16, CD19, CD33, CD34, CD44, CD56,CD57, CD99, CD117, TDT & HLADR. Cytogenetic was 46XY, +7, der(7;7)[10] and 46,idem,?del(6)(pterq21:)[5]/46,XY[5]. It revealed a male chromosome complement with two related abnormal cell lines. The (stem line) showed a dicenteric derivative chromosome 7 with possible uncertain inversion. The (sideline) showed the stem line abnormality complement with an uncertain deletion within the long arm of chromosome 6. FISH confirmed Isodicentric chromosome 7q. ECHO showed a small ASD secondum, multiple apical VSD, and compressed SVC with mild pericardial effusion, but normal left cardiac function. On further evaluation, he was found to have central hypothyroidism MRI showed normal pituitary with normal pituitary area on a cerebellar atrophy ((a,b)); therefore, he was started on levothyroxine by endocrine team.
Figure 1. CXR Pre and post Induction. Frontal chest x-ray images (a) at the time of diagnosis and (b) post induction. Large mediastinal opacity (star) is identified in the right Para tracheal of the superior mediastinum. Note also the right side pleural effusion (arrow). Resolution of the mediastinal mass on the chest x-ray post treatment.
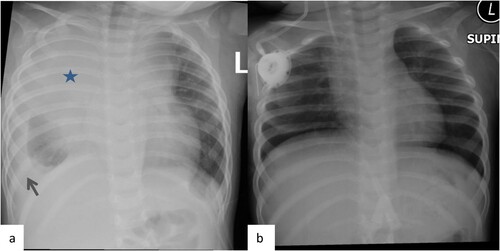
Figure 2. CT chestPre and post Induction. Selected axial images from the CT scan of the chest. (a)Trans-axial image from the non-enhanced CT scan of the chest at the time of diagnosis demonstrates the large right mediastinal soft tissue mass at the level of the heart. (b) trans-axial image from the contrast enhanced CT scan of the chest after the induction demonstrate the mediastinal mass with significant reduction in size.
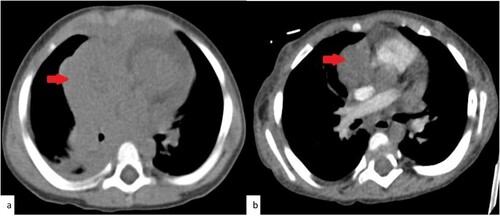
Figure 3. MRI brain Base line. Selected images from the MRI of the brain before the chemotherapy treatment. (a) Sagittal T1 weighted MR image at the midline demonstrate the mild cerebellar atrophy (arrow). (b, c) selected axial T2 weighted images of the supratentorial brain shows no evidence of white matter change.
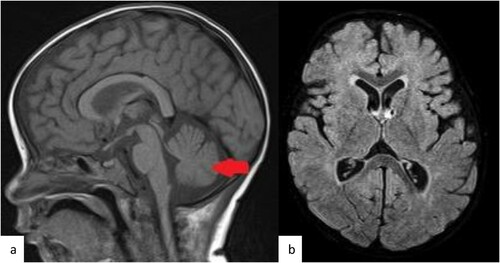
Table 1. Initial blood result.
Early diagnosis was made because of his early presentation as he presented with low IgG and had a positive family history of childhood lymphoma. Genetics and Immunology teams were involved. Underlying immunodeficiency was suspected; therefore, the patient was planned for 4weeks IVIG. The whole exome sequence was sent.
Management course
Patient started immediate chemotherapy as per local protocol CALL08 High risk Arm (C) [Citation4]. He completed unmodified induction and augmented consolidation phase. He achieved hematological and radiological remission post induction. Bone marrow flow cytometry (FC) on day 15 and 29, confirmed negative (MRD less than 0.01%). CXR and CT chest at the end of induction confirmed resolved mediastinal mass ((b) and (b)).
By end of the augmented consolidation phase, the whole exome sequence was consistent with the diagnosis of A-T; it detected a class1 pathogenic homozygous variant in ATM gene, c.9066del (p. Gly3023Alafs*10) it was the first time to be detected and so far not listed in Gento MD, both parents were heterozygous. Genetics counseling was done.
The patient’s diagnostic radiological exposure was minimized during his management course; his chemotherapy was downgraded to Arm B [Citation4] and modified as shown in (). The Interim Maintenance I (High dose Methotrexate) phase was replaced by Capizzi. During (IM) Capizzi, he tolerated the 3 escalating MTX doses (100→150→200 mg/m2) but did not tolerate the 4th dose (250 mg/m2). He developed grade III mucositis and prolonged fever neutropenia. Therefore, the 5th dose was omitted. In delayed intensification (DI)Doxorubicin was reduced by 20%. Nevertheless, we planned to reduce cyclophosphamide by 50% and to add Mesna. As we were concerned about the increasing risk of hemorrhagic cystitis due to recurrent hematuria, but due to his clinical condition cyclophosphamide was omitted in DI phase. Our patient completed 18/36 months of maintenance chemotherapy receiving Vincristine monthly and Intrathecal Methotrexate every 3 months; total of 23 intrathecal methotrexate. His daily oral Mercaptopurine and weekly oral Methotrexate started as 60% of total dose, and then was gradually upgraded reaching 100% of optimal dose.
Table 2. Chemotherapy modification.
Table 3. Grade 3 and above CTCAE Chemotherapy toxicity and modification
His chemotherapy was stopped at month 18 of Maintenance due to acute neurological progression likely related methotrexate and vincristine. The patient remained ventilator dependent with persistent slow improving GCS until his death one year after therapy discontinuation.
Comorbidities and toxicity
As shown in (), the patient developed CTCAE grade IIIon top of cytopenia and infection during his chemotherapy, in addition to Peg Asperginase related severe hypertriglyceridemia. His triglyceride level reached 22.9 mmol/L (Normal value 0.50–2.23 mmol/L). It resolved spontaneously on low-fat diet. By the end of the induction phase, he developed peripheral neuropathy as a result of Vincristine toxicity; it was resolved by the mid of the consolidation after a course of pyridostigmine and pyridoxine, vincristine dose was not reduced for the subsequent doses. Only during Cappizi and DI Modification was needed, when at the end of the 4th cycle of Capizzi, he had mucositis GI grade III with prolonged fever neutropenia. Therefore, his 5th Capizzi cycle was omitted. On DI phase, he developed recurrent hematuria with prolonged thrombocytopenia, in which he was admitted for clinical typhlitis and was managed conservatively.
During Maintenance phase, he was admitted three times due to mild to moderate chest infection and asthma exacerbations that resolved with IV antibiotics and steroids. At month 18 of Maintenance, we stopped his chemotherapy treatment because of acute neurological progression that happened 3 months after his Intrathecal Methotrexate and one month after Vincristine dose, as he was on full oral Methotrexate dose as well.
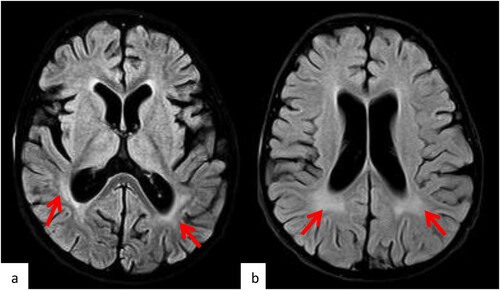
Initially, the patient was presented with decreased activity and progressive hypotonia (head lag, unable to sit, and UMN facial palsy with aphasia). His CSF was negative for relapse and infection which showed normal glucose and protein. EEG showed no epileptic discharges. However, brain MRI reported changes that were likely due to post chemotherapy effect; Persistent diffuse brain volume loss with prominent ventricular system and enlargement CSF space, bilateral mild white matter periventricular at the level of the occipital horn ((a & b)). Steroid, vincristine and methotrexate was on hold, he was continued on his monthly IVIG and started by neurology on Mitochondrial cocktail; biotin, carnitine, coenzyme10, vitamin C, E, B6 and B12
Figure 4. MRI brain Post neuro-toxicity. Selected axial FLAIR MR images (a, b) of the brain post chemotherapy demonstrate the development of the periventricular white matter changes (arrows) suggestive of the toxicity.
The patient then developed a progressive decrease level of consciousness (GCS 9 -12) and desaturated with acidosis; therefore, he required mechanical ventilation. After a multidisciplinary case conference, we decided to stop chemotherapy and keep him on supportive care management. Despite chemotherapy modification and close monitoring for myelotoxicity and the risk of secondary malignancy, he had an early and sudden rapid neuro-toxicity, most likely related to methotrexate and vincristine, which made him remain ventilator dependent with persistent low GCS 10, he remains ventilator dependent with slow improving GCS to12 until his death one Year after therapy discontinuation.
Discussion
Ataxia telangiectasia is a rare, autosomal dominant, inherited, and progressive neurodegenerative disease. It is characterized by immunodeficiency, sensitivity to ionizing radiation, genomic instability, and increased predisposition to cancer, especially to lymphoid malignancies [Citation5]. Its incidence of malignancy is 24.5% in the largest cohort [Citation6]. Malignancy can present early and can precede the diagnosis of A-T. Our patient and his brother were both presented with a very early onset of T cell neoplasm (less than 2 years) even before the symptoms of A-T appeared.
Serum alpha-fetoprotein is a simple, rapid, and diagnostic screening test for A-T; it is proven to be elevated (levels above 30 ng) in more than 90% of A-T cases. AFP level test was done for our patient in investigation for mediastinal mass. The result was high as it was high for his older brother as well. Our patient showed no typical symptoms of A-T. However, elevated levels of AFP raised our suspicions to make the diagnosis of A-T highly likely. Further investigations of genetic testing confirmed our diagnosis.
Chromosome abnormality involving chromosomes 7 and 14 are typically identified in individuals with A-T. Translocations with breakpoints are commonly at 14q11 (the T-cell receptor-alpha/delta locus) and at 14q32 (immunoglobulin heavy chain gene), 7p14 7q35 resulting in immunoglobulin/T-cell receptor gene trans- or cross-rearrangements. Other abnormality such as inversion of chromosome 7 or 14 was also reported [Citation7]. In our case, the cytogenetic showed (stem line) of dicentric derivative chromosome 7 with possible inversion. FISH confirmed Isodicentric chromosome 7q.
Due to lack of evidence-based protocol to follow, chemotherapy modification strategy remains datable, depending on the center’s experience in such cases. Many reports disclosed excessive and fatal toxicity as a result of radiotherapy treatment; therefore, there is a consensus to avoid craniospinal radiation therapy [Citation8].
As reported by Cohen et al., cyclophosphamide has shown to have serious side effects including toxic hemorrhagic cystitis (HC) and life-threatening bleeding due to bladder wall telangiectasia in A-T patients. It is usually severe with Cyclophosphamide dose above 1200 mg/m2 [Citation9]. Our patient developed grade II HC after the first dose of Cyclophosphamide at a dose of 1 gm/m2 and received no further dosages.
Some case reports advice to modify neurotoxic drugs (such as Vincristine) because A-T patients have decreased ability to compensate muscle weakness [Citation10]. It may be safer if vincristine is discontinued, delivered at a reduced dosage, or replaced by Vinblastine whenever early signs of muscle weakness are noticed. Our patient developed bilateral foot drop as a result of VCR toxicity which was managed with a course of pyridostigmine and pyridoxine. Maximum dose of Vincristine was then resumed after complete recovery with no further symptoms’ recurrence.
A-T patients are prone to methotrexate-induced gastrointestinal tract toxicity; therefore, it is of great benefit to optimize drug clearance methods. As for our patient, we elected to cancel High dose Methotrexate and replace it with one CAPIZZI. The dose of MTX was adjusted according to tolerance. There was no use of folinic acid rescue for IV or OT MTX.
Multiple case series have described the results of chemotherapy treatment of lymphoid malignancies in patients with A-T. In 1980, Sandoval reported a cohort of A-T patients who developed lymphoid malignancies (NHL in 64%, ALL in 15%, and HD in 13%). All patients receiving chemotherapy achieved complete remission with a survival rate of 6–46 months. One patient died due to progressive disease after two months of treatment using prednisolone as monotherapy. Patients who received a standard dose of chemotherapeutic treatment have shown better outcomes than modified induction [Citation11]. In our reported case, the patient received unmodified induction and consolidation due to the delay in the diagnosis of A-T. Although our patient did not develop sudden unusual severe toxicity, we elected to modify his chemotherapy based on most reported cases in the literature view as described above.
In addition to cytotoxic drugs, A-T patients experience radiotherapy treatment toxicity as well. In a systemic review by Schoenaker, 23 A-T cases were conducted and analyzed in which all achieved complete remission except one. Both modified and unmodified treatment strategies can induce complete remission; there’s no significant difference in achieving CR (94%). However, unmodified treatment is better tolerated in younger A-T patients than in older A-T patients. Unmodified treatment shows higher survival rates than modified treatment, 73% and 57% respectively. Upfront modified treatment events were related to toxicity and not caused by relapse. Modified treatment may lead to lower EFS; yet, it has less toxicity and better life quality. Apart from regular immunoglobulin replacement therapy, no additional supportive care is needed in A-T patients [Citation2]. Our Patient achieved CR after unmodified induction and maintained it with the chemotherapy modification. Apart from the acute neuro-toxicity, all other symptoms were manageable and reversible.
In conclusion, A-T should be suspected in any child who presents early with T cell ALL, family history, high AFP, and low immunoglobulins. Treatment of ALL in children with A-T is feasible as long as the diagnosis is made early. To prevent the child from toxic death and secondary malignancy due to unnecessary exposure to radio- and chemotherapeutic agents, early family genetic counseling is of great value. Nevertheless, unmodified induction treatment strategies are successful to induce complete remission. Treatment can be modified according to the patient’s clinical condition, immune deficiency status, beside tolerance, and type of toxicity developed. Despite the modification of chemotherapy and the close monitoring for myelotoxicity, the patient may develop acute methotrexate and vincristine-related neuro-toxicity. Neuro-toxicity can be avoided by either folinic acid rescue or modification of the Vincristine vs Vinblastine dosage prophylactically. May be the prolonged maintenance treatment including vincristine pulses associated with sudden severe and fatal neuro-toxicity. Further evidence-based medicine is strongly needed to elucidate the optimal treatment strategy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Taylor M. Ataxia-telangiectasia in children, guidance on diagnosis and clinical care. Nottingham University Hospitals. The Ataxia-Telangiectasia Society; 2014.
- Crawford TO, Skolasky RL, Fernandez R, et al. Survival probability in ataxia telangiectasia 2006 Archives of Disease in Childhood.
- Szczepanski T, Mahlaoui N, Loeffen JL. Treatment of acute leukemia in children with ataxia telangiectasia/M.H.D. Schoenaker et al. Eur J Med Genet. 2016;59:641e646.
- Thomas A, Waldmann K. Robert Mcintire, serum-alpha-fetoprotein levels in patients with ataxia-telangiectasia Metabolism Branch and Laboratory of Cell Biology. Bethesda, Maryland 20014, U.S.A., the lancet Nov 25: National Cancer Institute; 1972.
- Jastaniah W, Elimam N, Abdalla K, et al. Felimban s early vs. late MRD response- and risk-based treatment intensification of childhood acute lymphoblastic leukemia: a prospective pilot study from Saudi Arabia. Exp Hematol Oncol. 2018 Nov 19;7:29, doi:https://doi.org/10.1186/s40164-018-0121-x. . e Collection 2018.PMID:30479872.
- Taylor AM, Metcalfe JA, Thick J. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996;87:423–438.
- Suarez F, Mahlaoui N, Canioni D, et al. Incidence, presentation, and prognosis of malignancies in ataxia-telangiectasia: a report from the French national registry of primary immune deficiencies. J Clin Oncol. 2015;33–2(10):202e208.
- Gatti R. Ataxia-telangiectasia. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Genereviews® [internet]. Seattle, WA: University of Washington; 1993-2016.
- Yanofsky RA, Seshia SS, Dawson AJ, et al. Ataxia-telangiectasia: atypical presentation and toxicity of cancer treatment. Can J Neurol Sci. 2009;36(4):462e467.
- Cohen JM, Cuckow P, Davies EG. Bladder wall telangiectasia causing life-threatening hematuria in ataxia-telangiectasia: a new observation. ActaPaediatr. 2008 May;97(5):667e669.
- Pietrucha1 B, Heropolitañska-Pliszka E, Gatt RA, et al. Ataxia-Telangiectasia: guidelines for diagnosis and comprehensive care. Los Angeles: The David Geffen School of Medicine at UCLA; 2007.
- Sandoval C, Swift M. Treatment of lymphoid malignancies in patients with ataxia-telangiectasia. Med Pediatr Oncol. 1998 Dec;31(6):491e497.
