ABSTRACT
Objectives
The primary objective was to compare the efficacy of intermediate-dose cytarabine (IDAC) and high-dose cytarabine (HiDAC) as consolidation chemotherapy for acute myeloid leukemia (AML) in terms of a one-year-relapse-free survival rate (RFS). The secondary objectives were one-year-overall survival rate (OS) and adverse effects.
Methods
This was a retrospective study conducted at Chiang Mai University Hospital. AML patients who achieved complete remission after 7 + 3 induction regimen and received consolidation therapy with either IDAC or HiDAC during January 2015 and January 2018 were eligible. Data about clinical characteristics, efficacy and safety of IDAC and HiDAC regimens were collected.
Results
Sixty-two AML patients were enrolled (30 patients in IDAC and 32 patients in the HiDAC regimen). The one-year RFS in the IDAC group was 63.33% and 46.87% in the HiDAC group (P = 0.137). The 1-year OS was 93.33% and 84.37% in the IDAC and HiDAC, respectively (P = 0.691). The duration of grade 3–4 thrombocytopenia was significantly shorter in IDAC than HiDAC (mean duration 14.69 vs. 23.84 days; P = 0.045). There was no significant difference in other parameters including hemoglobin nadir, absolute neutrophil count nadir, platelet nadir, febrile neutropenia, duration of grade 3–4 neutropenia, and duration of hospitalization.
Discussion and conclusions
There was no significant difference in the one-year RFS and OS between IDAC and HiDAC. The IDAC regimen is an acceptable option for consolidation treatment in AML.
Introduction
Acute myeloid leukemia (AML) is a hematologic cancer resulting from the clonal expansion of myeloid blasts in the bone marrow (BM), peripheral blood, or other tissues [Citation1]. The worldwide incidence rates of AML range from 2.5 to 3 cases per 100,000 populations per year [Citation1]. In United States, estimated 21,450 AML patients were diagnosed in 2019 [Citation2]. The large registry from Sweden showed the incidence of AML increased with age with the median age of diagnosis of 72 years [Citation3]. The diagnosis of AML according to the 2016 World Health Organization (WHO) Classification requires at least 20% of myeloblasts, monoblasts/promonocytes, and/or megakaryoblasts in the peripheral blood or BM [Citation1]. Cytogenetic analysis from BM is a recommended initial investigation to classify the overall prognosis and guide in determining treatment [Citation4–6]. Cytogenetic risks can be divided into three groups including favorable risk, intermediate-risk, and adverse risk [Citation4,Citation5].
Specific treatment of AML in patients who are eligible for intensive chemotherapy includes induction and post-remission therapy [Citation4–6]. Induction treatment is a therapy to induce the remission of the disease and is given to achieve the goal of complete remission (CR) [Citation4–6]. The regimen called ‘7 + 3’ is composed of cytarabine given continuously via intravenous infusion (CIV) for seven days and anthracycline (such as daunorubicin and idarubicin) intravenously for three days [Citation7] is the mainstay of induction chemotherapy [Citation4–6].
Patients with AML who achieved CR require a post-remission therapy to eradicate any residual disease and to prevent relapse [Citation4–6]. The consolidation chemotherapy for three to four cycles is recommended in patients with favorable or intermediate cytogenetic risk. However, patients with adverse risk and selected patients in the intermediate-risk group should undergo allogeneic stem cell transplantation (allo-SCT) [Citation4–6]. The widely used consolidation regimen is high dose cytarabine (HiDAC; 3 g/m2 every 12 h for 3 days) that has demonstrated efficacy in terms of disease-free survival (DFS) and overall survival (OS) over standard-dose cytarabine (SDAC; 100 and 400 mg/m2 CIV for 5 days) in randomized controlled trial (RCT) since the year 1994 [Citation8]. Recently, studies comparing intermediate-dose cytarabine (IDAC; 1–2 g/m2 every 12 h for 3 days) to HiDAC during consolidation therapy found no significant differences in efficacy. Besides, IDAC seemed to have fewer side effects [Citation9,Citation10]. The meta-analysis also found no significant difference in DFS in five years between HiDAC and IDAC, with an HR of 0.93 (95% CI, 0.83–1.05, P = 0.24) [Citation11]. As a result, IDAC becomes an acceptable post-remission consolidation therapy and is recommended by the European LeukemiaNet (ELN) guidelines [Citation5].
Chiang Mai University Hospital, Thailand has implemented IDAC as a consolidation therapy since January 2017 for the majority of AML cases who achieved CR after induction therapy including patients who had intermediate or adverse risk cytogenetic and could not be treated by allo-SCT due to the limitation on medical expense coverage scheme or inability to find a human leukocyte antigen (HLA) matched donor. This scheme of treatment was based on the European LeukemiaNet guidelines [Citation5] but it has not been thoroughly evaluated. This study aims to compare the efficacy of IDAC and HiDAC for the treatment of AML during consolidation in real-world practice.
Materials and methods
Study overview
This study was a retrospective study conducted at Chiang Mai University Hospital, Thailand. Patients diagnosed with AML from January 2014 to January 2019, 18–60 years of age, who achieved CR after induction chemotherapy with standard ‘7 + 3’ regimen and received consolidation chemotherapy with HiDAC or IDAC for at least one cycle were enrolled. Exclusion criteria were AML patients who had been treated with allo-SCT, pregnancy or lactating, had second malignant neoplasms, human immunodeficiency virus (HIV) infection, and patients with acute promyelocytic leukemia. Data collection was performed by reviewing patients’ medical records. Clinical and laboratory features including age, gender, Eastern Cooperative Oncology Group (ECOG) performance status, complete blood count (CBC), number of blast cells in blood and BM, lactate dehydrogenase (LDH) level, and cytogenetic results were collected. FLT3-ITD mutation was only available molecular testing in the institute since September 2016 and was analyzed in all AML patients before induction and re-tested only in FLT3-ITD mutated patients before each consolidation therapy. The detection of minimal residual disease (MRD) for other AML-associated genetic lesions was not available. Number of chemotherapy cycles and the dosage of cytarabine were also recorded.
The primary objective was to compare the efficacy of IDAC and HiDAC in terms of a one-year relapse-free survival (RFS), defined as the proportion of patients who achieved CR until relapse or death from any cause [Citation5]. The secondary objectives were to compare one-year OS and adverse effects of IDAC and HiDAC. OS was measured from the date of diagnosis to the date of death from any cause [Citation5]. Response criteria were based on ELN guidelines [Citation5]. Toxicity was analyzed according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [Citation12] except for bleeding events. The bleeding adverse events are classified into three groups: (1) ‘None’ represents a group of subjects who has no bleeding; (2) ‘Minor bleeding’ is a group of subjects who have bleeding symptom with improvement after medical treatment, and (3) ‘Major bleeding’ is a group of subjects who have severe or life-threatening bleeding and require blood component transfusion plus any surgical intervention [Citation13]. This study was approved by the Institutional Research Committee of the Faculty of Medicine, Chiang Mai University and was conducted in accordance with the Declaration of Helsinki.
Treatment protocol
Induction therapy as a ‘7 + 3’ regimen included cytarabine 100 mg/m2 CIV for seven days and intravenous idarubicin 12 mg/m2 for three days [Citation5]. Second induction therapy with the same regimen was administered in patients who achieved partial remission (PR) [Citation5]. Patients who achieved CR [Citation5] after 1–2 cycles of induction before January 2017 received HiDAC (cytarabine 3 g/m2 every 12 h on day 1, 3, and 5) [Citation8] for four cycles as consolidation therapy, while patients after January 2017 received IDAC (cytarabine 1.5 g/m2 every 12 h on day 1, 3, and 5) [Citation10]. The courses of consolidation therapies might decrease to 1–3 cycles in patients who experienced life-threatening neutropenic sepsis according to the attending physician’s decision. Granulocyte-colony stimulating factor (G-CSF) prophylaxis was prescribed in all cases during consolidation therapy started at day six until ANC was more than 1 × 109/L. Blood components were administered according to the institute’s protocol. Infectious prophylaxis included oral acyclovir 400 mg twice daily and oral itraconazole 200 mg twice daily for herpes zoster and fungal prophylaxis, respectively. Itraconazole was used instead of posaconazole because of the inaccessibility under medical expense coverage scheme. Febrile neutropenia was treated according to international guidelines [Citation14].
Statistical analysis
From the prospective RCT that compared HiDAC and IDAC during consolidation therapy found no significant difference in the five-year DFS (38% [95% CI, 31% to 45%] vs. 37% [95% CI, 31% to 44%], P = 0.86). The DFS in one year for HiDAC and IDAC was estimated at 70% and 60%, respectively [Citation9]. When using the statistical calculation by two-sample proportions to calculate the sample size for this study and the power of the test set at 0.8, the estimated sample size was 300 patients per group. Due to an insufficient sample size of AML patients in Chiang Mai University Hospital, the researcher decided to set sample size per arm at least 30 patients (10% of the project sample size) which reference from pilot study theory.
Baseline characteristics were described using descriptive statistics. Univariable comparisons were performed by the independent t-test for continuous variables and by Pearson Chi-square or Fischer exact test for categorical variables. Statistical significance level sets when the P-value < 0.05. Survivals were plotted by using the Kaplan–Meier method and compared by the log-rank test and univariate proportional hazards analysis. The Statistical Package for the Social Sciences (SPSS) software was used for statistical analyses (Version 23.0; SPSS Inc., IBM Corp, Armonk, NY, USA; 2015).
Results
Baseline characteristics
During the study period, a total of 62 patients met the inclusion criteria and were enrolled in this study. Among these patients, 30 patients were treated with the IDAC regimen and 32 patients were treated with a HiDAC regimen.
The baseline characteristics of patients in both groups were generally not significantly different (). Most patients were females (73.33% in IDAC group vs. 62.50% in the HiDAC group) with a median age of 42.50 (range 20–63) and 38.50 (range 18–60) years in the IDAC and HiDAC groups respectively. Approximately 80% of patients in both groups (83.33% in IDAC and 81.25% in the HiDAC group) received only one cycle of induction chemotherapy, while the others received two inductions because they achieved only PR from the first induction. About half of patients in both groups had an ECOG performance status of one before the treatment (53.33% in the IDAC group vs. 53.13% in the HiDAC group).
Table 1. Baseline characteristics of patients (n = 62).
According to the classification of AML, AML without/with maturation was the predominant subtype in both groups, followed by acute myelomonocytic leukemia. Regarding cytogenetic risk, most of the patients were classified as intermediate risk (63.33% in the IDAC vs. 65.63% in the HiDAC group), followed by adverse risk (30% in IDAC vs. 25% in HiDAC group).
Most of the patients received three to four cycles of consolidation chemotherapy. In the IDAC group, 23.33% of the patient received three cycles and 63.33% received four cycles. In the HiDAC group, 53.13% of patients received three cycles and 25.00% of patients received four cycles (P = 0.024). The remainder of the patients relapsed or died before receiving the target three to four cycles of chemotherapy. There was no patient who required cytarabine dose modification from HiDAC to IDAC or vice versa. The mean dose of cytarabine in the IDAC group was 1.49 g/m2 and in the HiDAC group was 2.86 g/m2 per dose.
Primary outcome: relapse-free survival
The overall one-year RFS for all populations was 54.84%. (34 from 62 patients) One-year RFS in the IDAC group was 63.33% (19 from 30 patients) and the HIDAC group was 46.87%. (15 from 32 patients) (P = 0.137) ().
Figure 1. Kaplan–Meier curves of 1-year relapse-free survival of acute myeloid leukemia patients in intermediate-dose cytarabine (IDAC) and high dose cytarabine (HiDAC) groups.
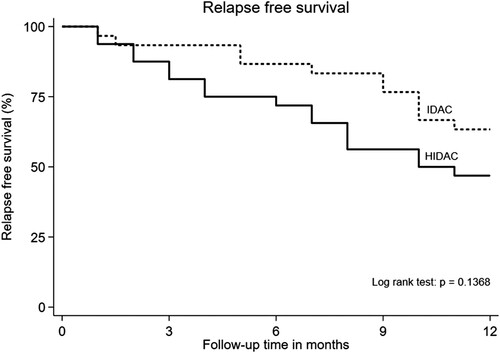
Considering RFS in a subgroup analysis of cytogenetic risk, we found that in a group of favorable cytogenetic risk, both IDAC and HiDAC provided excellent treatment results with no relapse found within one year (1-year RFS 100% in both groups). RFS in a group of intermediate cytogenetic risk had no significant differences between IDAC and HiDAC (one-year RFS in IDAC 63.2% vs. HiDAC 42.9%, P = 0.30). The group of adverse risk had the highest relapse rate when compared to other groups. However, there is no statistically significant difference in one-year RFS between the IDAC and HiDAC group (55.6% vs. 37.5%, P = 0.18). The analysis of RFS according to cycles of consolidation therapy revealed IDAC three cycles, IDAC four cycles, and HiDAC three cycles tended to have better one-year RFS compare to four cycles of HiDAC. (71.43%, 73.68%, 70.59% vs. 37.50%, respectively).
Secondary outcomes
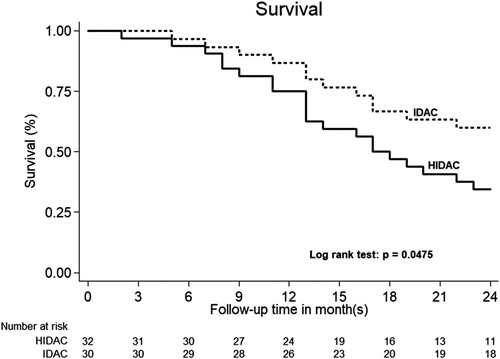
The one-year OS was 93.33% in the IDAC group and 84.37% in the HiDAC group with no significant statistical differences (P = 0.691) (). After the median follow-up time of 22 months (IQR 13–37 months), the two-year RFS are 56.7% in IDAC group and 25.0% in HDAC group and P = 0.014 (). The cumulative incidence of relapse in IDAC group was 43.3% (95% CI = 25.5–62.6%), while and the cumulative incidence of relapse in HiDAC group was 75% (95% CI = 56.6–88.5%) (P = 0.014). Non-relapse mortality is not found in both IDAC and HiDAC groups. The two-year OS was 60.0% in IDAC and 34.4% in HiDAC group (P = 0.047) ().
Figure 2. Kaplan–Meier curves according to the 1-year overall survival of acute myeloid leukemia patients in intermediate-dose cytarabine (IDAC) and high dose cytarabine (HiDAC) groups.
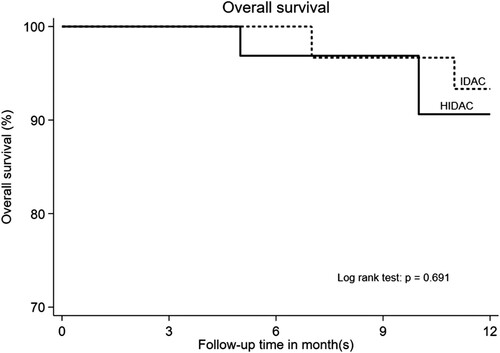
Figure 3. Kaplan–Meier curves of 2-year relapse-free survival of acute myeloid leukemia patients in intermediate-dose cytarabine (IDAC) and high dose cytarabine (HiDAC) groups.
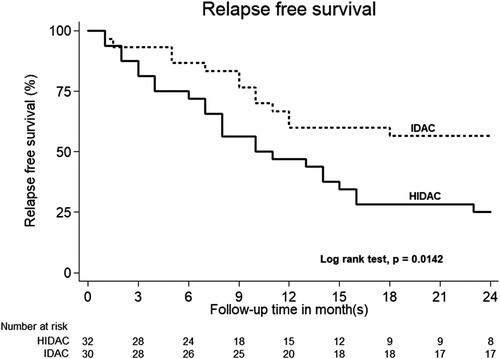
Figure 4. Kaplan–Meier curves according to the 2-year overall survival of acute myeloid leukemia patients in intermediate-dose cytarabine (IDAC) and high dose cytarabine (HiDAC) groups.
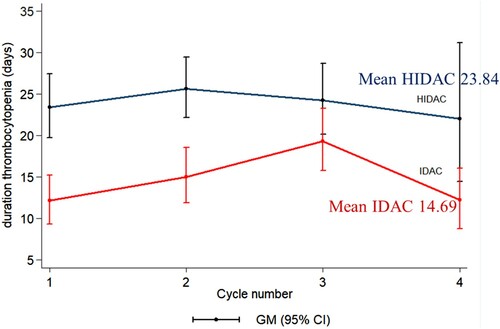
Regarding hematologic adverse events, there were no significant differences in parameters including mean hemoglobin (Hb) nadir, ANC nadir, platelet nadir compares between the IDAC and HiDAC group. However, the mean ANC nadir in the IDAC group tended to be lower than the HiDAC group (mean ANC 0.008 × 109/L and 0.016 × 109/L, respectively; P = 0.058). The duration of grade three to four thrombocytopenia was significantly lower in the IDAC group than the HiDAC group (mean 14.69 and 23.84 days, respectively; P = 0.045) (). There was no significant difference in other parameters including the duration of grades 3–4 neutropenia and grade 4 neutropenia. In terms of duration of hospitalization, the IDAC group tended to have shorter hospitalization days than the HiDAC group but no statistical difference (mean 21.87 days vs. 23.19 days; P = 0.055). There is no difference in the time periods that patients in each group received consolidation chemotherapy (geometric means for IDAC vs. HiDAC were 39.0 days vs. 40.4 days for cycle 1 [P = 0.544], 40.5 days vs. 44.1 days for cycle 2 [P = 0.108], and 38.3 days vs. 40.9 days for cycle 3 [P = 0.459]).
Figure 5. Comparison of duration of Grades 3–4 thrombocytopenia in each cycle between intermediate dose (IDAC) and high dose cytarabine (HiDAC) consolidation chemotherapy. (P = 0.045); GM: geometric mean.
represented the grading of adverse events in each cycle of IDAC and HiDAC. More than 70% of the patients had grade 3 anemia, 80–90% had grade 3 febrile neutropenia, and 100% had grade 4 thrombocytopenia during consolidation therapy and there were no significant differences between the IDAC and HiDAC group. Major bleeding occurred only in the HiDAC group (less than 5%). Minor bleeding was found at about 40% and there were no significant differences in bleeding events between IDAC and HiDAC groups (P = 0.363).
Table 2. Adverse events in each cycle of high-dose and intermediate-dose cytarabine for consolidation therapy.
According to non-hematologic adverse events, grade 1–3 nausea and vomiting and diarrhea occurred in about 10-20% which was not a significant difference between the two groups (P = 0.902 and P = 0.460). Allergic reaction grade 1–3 tended to occur consistently lower in every cycle of IDAC than HiDAC (21.32% vs. 29.56%, respectively) but it was not statistically significant (P = 0.911). Finally, grades 2 and 3 cardiac adverse events were found only in the HiDAC group (grade 2 cardiac event 3.13% and grade 3 cardiac event 7.13%) while none in the IDAC group. However, there was no significant difference (P = 1.00).
Discussion
Since the RCT conducted by the Cancer and Leukemia group B (CALGB) in 1994, HiDAC became standard consolidation chemotherapy for AML [Citation8] especially in favorable and intermediate-risk patients who did not undergo allo-SCT [Citation15]. However, recent studies compared HiDAC with the IDAC regimen for the consolidation treatment of AML and found no significant differences in RFS between both dosages, and IDAC seemed to have fewer side effects [Citation9,Citation10]. As a result, the ELN guideline recommended IDAC as acceptable post-remission consolidation therapy in favorable and intermediate-risk genetics since 2017 [Citation5]. There is some consideration regarding the application of this recommendation into clinical practice in Thailand since the majority of patients cannot access allo-SCT [Citation16] including those who harbored adverse risk cytogenetic as well as differences in induction and consolidation regimens in previous IDAC trials [Citation9,Citation10]. As a result, real-world data about IDAC for consolidation therapy after the standard 7 + 3 induction is needed. This study chose one-year RFS and OS as main endpoints because the IDAC regimen was applied to AML patients in our institute after January 2017 and the authors aimed to detect the difference between IDAC and HiDAC as early as possible.
In our study, the mean of cytarabine dosage was 1.49 g/m2 in the IDAC group and 2.86 g/m2 in the HiDAC group. These values are close to the theoretical definition of IDAC and HiDAC doses which are 1–2 g/m2 and 2–3 g/m2, respectively. Primary and secondary outcomes regarding the efficacy of the treatment revealed no significant difference in the one-year RFS and OS between patients who received either IDAC or HiDAC. However, the two-year RFS and OS in the IDAC group tended to be higher than HiDAC group. For safety profiles, patients who received IDAC had a significantly shorter duration of grade 3–4 thrombocytopenia.
Regarding the Medical Research Council (MRC) AML15 study [Citation10], the induction course in AML15 was different from our study. Combination therapy with daunorubicin, cytarabine (DA), with or without etoposide (ADE) or daunorubicin and cytarabine or fludarabine, cytarabine, G-CSF, and idarubicin (FLAG-Ida) regimen for two cycles were used instead of the standard 7 + 3 regimen as in our study. In the consolidation phase, patients were randomly assigned to receive HiDAC, IDAC (3 and 1.5 g/m2 every 12 h for 3 days, respectively) or amsacrine, cytarabine, etoposide, and mitoxantrone/cytarabine (MACE/MidAc). The dose of HiDAC and IDAC were similar to our study but administered only for two or three cycles. Although the OS was not different and IDAC required less supportive care as well as shorter hospitalization than HiDAC, there was a trend for a higher relapse risk in the IDAC arm (eight-year RFS of 34% in IDAC vs. 42% in HiDAC; P = 0.100). Our study confirmed these findings except we did not observe the trend of higher relapse in the IDAC group at one year across all cytogenetic subgroups. Moreover, the two-year RFS and OS in the IDAC group were higher than HiDAC group. The one-year RFS of overall patients and in the IDAC group of 54.84% and 63.33%, respectively, in our study was comparable with the outcomes in AML15 study [Citation10] and CALGB study [Citation8] (approximately 60%). In contrast, the one-year RFS of 46.87% in the HiDAC group in our study was lower than expected [Citation8,Citation10]. The imbalance in total cycles of IDAC (63.33% received four cycles) and HiDAC (53.13% received three cycles) group was observed and is concerned. The early termination of HiDAC cycles might be from a prolonged duration of thrombocytopenia or serious complications even it did not result in mortality. The improvement of supportive care during time periods might also contribute to higher proportion of patients who received 4 cycles of IDAC since IDAC was adopted later and could lead to bias. However, we could not demonstrate the effect of the number of cycles since IDAC for three cycles still had tended toward better RFS than HiDAC for the four cycles group. The worst RFS of four cycles of HiDAC group could be interpreted with caution because of limited number of patients in each subgroup.
Another trial, the Study Alliance Leukemia (SAL) AML96 study [Citation9] aimed to compare consolidation therapy with HiDAC (3 g/m2 every 12 h for 6 days) and IDAC (1 g/m2 every 12 h for 6 days) after double induction with cytarabine, mitoxantrone, etoposide, and amsacrine (MAV and MAMAC). There were no significant differences in the five-year DFS (37% vs. 38%; P = 0.860) and OS (30% vs. 33%; P = 0.770). The IDAC arm had a shorter duration of neutropenia and required blood transfusion less than HiDAC. The comparison between AML96 study and our study must be interpreted with caution because the different induction and consolidation regimens (six days duration with combination with mitoxantrone to both IDAC and HiDAC and given for one cycle) were used. Moreover, further treatments according to cytogenetic risk including other chemotherapy, autologous, or allo-SCT were planned in the AML96 trial but not included in our study.
There were controversial issues regarding using IDAC as consolidation therapy in all AML patients. The analysis from CALGB study [Citation17], Japan Adult Leukemia Study Group (JALSG) AML201 study [Citation18], a retrospective study [Citation19] and meta-analysis [Citation11] supported the use of HiDAC in favorable risk cytogenetic patients including core-binding factor AML at least in terms of DFS or RFS. However, the study of CALGB and JALSG AML201 was designed to compare HiDAC and SDAC (with [Citation18] or without other chemotherapy [Citation8,Citation17]), not directly comparable to IDAC. While the retrospective study in AML with t (8;21) revealed the RFS and OS benefit of HiDAC over IDAC and SDAC [Citation19], this study could not provide adequate information about this aspect because there were only five AML patients with favorable cytogenetic.
The benefit of the combination of other chemotherapy to IDAC during the consolidation phase was not demonstrated in clinical trials. The AML2003 RCT showed that IDAC in combination with mitoxantrone and amsacrine resulted in similar DFS and OS with HiDAC but had more toxicity [Citation20]. The corresponding figure was also shown in the retrospective study of mitoxantrone or idarubicin in addition to IDAC in patients with AML [Citation21,Citation22].
A limitation of this study came from the nature of the retrospective study design with the data of the study population obtained by medical chart review. Therefore, missing data and selection bias inevitably occurred. The data about units of packed red cells and platelet transfusion in each group could not be precisely gathered and compared. Also, no data about genetic mutation other than FLT3-ITD mutation was noted in this study. Importantly, the limited number of patients and a short follow-up period also limited the detection of the differences of RFS including subgroup analyses [Citation9,Citation10]. These problems may be solved by conducting a prospective study in a larger group of patients with longer follow-up.
Conclusions
There was no significant difference in the one-year RFS and OS between AML patients who received either IDAC or HiDAC as consolidation therapy. Patients who received IDAC had a significantly lower duration of grade 3–4 thrombocytopenia. This study supports the IDAC regimen as an acceptable option for consolidation treatment in AML. A study in a larger group of patients and longer follow-up are warranted.
Acknowledgements
The authors would like to thank Ms Antika Wongthani for suggestions in the statistics of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Arber DA, Orazi A, Hasserjian RP, et al. Introduction and overview of the classification of myeloid neoplasms. In: Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumors of haematopoietic and lymphoid tissues, 2017 revised edition. Lyon: International Agency for Research on cancer (IARC); 2017. p. 16–27.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer. J Clin. 2019;69:7–34. doi:https://doi.org/10.3322/caac.21551.
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. DOI:https://doi.org/10.1182/blood-2008-07-172007.
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441, DOI:https://doi.org/10.1038/bcj.2016.50.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. DOI:https://doi.org/10.1182/blood-2016-08-733196.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia version 3.2019. J Natl Compr Canc Netw. 2019;17:721–749. DOI:https://doi.org/10.6004/jnccn.2019.0028.
- Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:13;1249–1259. DOI:https://doi.org/10.1056/NEJMoa0904544.
- Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903.
- Schaich M, Rollig C, Soucek S M, et al. Cytarabine dose of 36 g/m2 compared with 12 g/m2 within first consolidation in acute myeloid leukemia: results of patients enrolled onto the prospective randomized AML96 study. J Clin Oncol. 2011;29:2696–2702. DOI:https://doi.org/10.1200/JCO.2010.33.7303.
- Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–3368. DOI:https://doi.org/10.1200/JCO.2012.47.4874.
- Wu D, Duan C, Chen L, et al. Efficacy and safety of different doses of cytarabine in consolidation therapy for adult acute myeloid leukemia patients: a network meta-analysis. Sci Rep. 2017;7:9509, DOI:https://doi.org/10.1038/s41598-017-10368-0.
- U.S. Department of Health and Human Service. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 November 27, 2017 [cited 2018 April 13]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
- Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–e93. DOI:https://doi.org/10.1093/cid/cir073.
- Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. DOI:https://doi.org/10.1001/jama.2009.813.
- Owattanapanich W, Utchariyaprasit E, Tantiworawit A, et al. Improved survival of elderly-fit patients with acute myeloid leukemia requiring intensive therapy: 3-year multicenter analysis from TALWG. Clin Lymphoma Myeloma Leuk. 2018: e509–e514. DOI:https://doi.org/10.1016/j.clml. cited 2018.08.002.
- Bloomfield CD, Lawrence D, Byrd JC, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179.
- Miyawaki S, Ohtake S, Fujisawa S, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 study. Blood. 2011;117:2366–2372. DOI:https://doi.org/10.1182/blood-2010-07-295279.
- Gong D, Li W, Hu LD, et al. Comparison of clinical efficacy of cytarabine with different regimens in postremission consolidation therapy for adult t(8;21) AML patients: A multicenter retrospective study in China. Acta Haematol. 2016;136:201–209. DOI:https://doi.org/10.1159/000448209.
- Schaich M, Parmentier S, Kramer M, et al. High-dose cytarabine consolidation with or without additional amsacrine and mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. J Clin Oncol. 2013;31:2094–2102. DOI:https://doi.org/10.1200/JCO.2012.46.4743.
- Zhou JH, Lin HQ, Shen Q, et al. Comparison of mitoxantrone in combination with intermediate-dose cytarabine versus high-dose cytarabine as consolidation therapies for young non-APL acute myeloid leukemia patients with favorable and intermediate cytogenetics. Curr Med Sci. 2018;38:51–57. DOI:https://doi.org/10.1007/s11596-018-1845-x.
- Kim DS, Kang KW, Lee SR, et al. Comparison of consolidation strategies in acute myeloid leukemia: high-dose cytarabine alone versus intermediate-dose cytarabine combined with anthracyclines. Ann Hematol. 2015;94:1485–1492. DOI:https://doi.org/10.1007/s00277-015-2389-9.
