ABSTRACT
Objectives
Treating red blood cells (RBCs) with dithiothreitol (DTT) is a wildly-recommended to overcome the interference of the daratumumab (DARA) with blood compatibility testing. Nevertheless, DTT can be hard to obtain in the clinical laboratory, while its use in routine practice may be time-consuming. In the following study, we explored the feasibility of using a commercial 2-mercaptoethanol (2-ME) working solution or the time-saving Polybrene method to mitigate DARA interference.
Methods
Antibody screening and cross-matching were performed using 2-ME or DTT-based indirect antiglobulin tests (IATs) and Polybrene method (with human IgG anti-E same IATs titer as DARA as positive control) on 37 samples. Most clinically important blood group antigens on RBCs were detected after treatment with 2-ME or DTT.
Results
Treating RBCs with 2-ME eliminates the DARA interference with the antibody screening or cross-matching; yet, K antigen is denatured during treatment. DARA does not interfere with antibody screening and cross-matching via Polybrene method, while 2+ agglutinations of anti-E antibody with the same titer (IATs method) as DARA could be observed in the positive controls via this method.
Conclusion
2-ME-based IATs or Polybrene method could replace DTT-based IATs to mitigate DARA interference.
Introduction
CD38 molecule is a type II transmembrane glycoprotein that highly expressed in hematological malignancies, including multiple myeloma (MM). Daratumumab (DARA) is an immunoglobulin G1κ (IgG1κ) full-human CD38 monoclonal antibody that specifically binds to CD38 antigen [Citation1]. However, considering that CD38 is also expressed on red blood cells (RBCs), although at a low level, the drug may also bind to healthy cells producing panreactivity in pre-transfusion compatibility testing [Citation2–4]. It has been found that significant panreactivity occurs on pre-transfusion immunohaematology testing after the clinical use of DARA [Citation5,Citation6]; thus, handling samples from patients treated with this drug requires a special strategy.
Currently, several methods, including the application of sulfhydryl reagents (e.g. dithiothreitol, DTT [Citation7,Citation8]), Polybrene [Citation9] and proteolytic enzymes (trypsin/papain) [Citation10,Citation11], DARA F(ab´)2 fragments [Citation12] and Fab fragments [Citation13], RBC phenotyping, RBC genotyping, and neutralizing proteins (anti-antibodies and soluble CD38 protein) have been reported to overcome the interference of DARA on transfusion compatibility testing. Treatment of RBCs with DTT (0.2M) is the most common method to remove DARA interference. As a thiol reducing agent, DTT can cleave disulfide bonds in the extracellular region of CD38 molecules so as to denature CD38 antigen and prevent it from binding to the CD38 antibody. Treatment of RBCs with DTT provides a safe blood supply for patients receiving DARA therapy [Citation7]. However, in some countries such as China, DTT is not conventionally used in clinic. On the other hand, 2-mercaptoethanol (2-ME) (0.2M), the same sulfhydryl reducing agent, is routinely used and easily available in China. Chapuy et al. suggested that the use of 2-ME might remove the interference induced by DARA [Citation8]; yet, it has not been confirmed by experiments.
Polybrene is a quaternary ammonium polymer which could cause non-specific RBC aggregation [Citation14]. Then specific aggregation is detected by adding a dilute sodium citrate-glucose solution to neutralize Polybrene effect on the cells. The Polybrene method is a simple and quick way for the detection of RBC antibodies with a high sensitivity except for the Kell system [Citation14,Citation15]. Since the Kell system has very limited clinical significance in east Asian population, the Polybrene method has been used in many clinical laboratories or blood banks in these areas [Citation16] and it has shown comparable results with standard pretransfusion tests [Citation15]. It was reported that the Polybrene method avoided interference of DARA on the compatibility of blood transfusion [Citation9,Citation17]; however, there is still a lack of large-sample, multi-center research and demonstration.
In this study, in order to verify the effectiveness of 2-ME and Polybrene methods, we treated RBCs of blood donors with 2-ME (0.2M) so as to avoid the interference of DARA; meanwhile, we performed a transfusion compatibility test with the Polybrene method in 24 patients with MM using DARA.
Materials and methods
Patients
A total of 24 DARA-treated MM patients were involved in this study. Among these patients, 12 patients were admitted to Changzheng Hospital, 5 Ruijin Hospital and 7 patients’ samples were sent to the blood group reference laboratory of Shanghai Blood Center between March 2018 and September 2020. All patients received one or more than one blood transfusion (total 37 times).
Tests before the first transfusion of DARA-received patient
Antibody screening tests for MM patients receiving DARA were performed with an IH-1000 automatic blood grouping analyzer (BIO-RAD, Roanne, France) or ORTHO Vision Max analyzer (Ortho, Mannedorf, Switzerland) for ABO, D blood group, and indirect antiglobulin tests (IATs).
DTT or 2-ME treatment of RBCs
The DTT (Roche, Mannheim, Germany) treatment of RBCs was carried out following the AABB technical manual [Citation18]. When treating donor RBCs with DTT, 2-ME was also used to treat the same cells, in order to compare the treatment effects between these two reagents. Two different brands of 2-ME working solution (Baso, Zhuhai, China; SHPBC, Shanghai, China) were used for RBCs, including antibody screening cells (SHPBC, Shanghai), panel RBCs (Sanquin, Amsterdam, Netherlands), single RBCs from blood donors. The 2-ME treatment of antibody screening RBCs, antibody identification panel cells, or donor RBCs was performed as follows: a total of 200 µL of 3% RBC suspension were washed four times with PBS (pH 7.4) before adding 800 μL of 2-ME in each tube. The mixture was incubated at 37°C for 30 min, mixing by inversion every 7–8 min. The cells were then washed four times with PBS (pH 7.4) and re-suspended in 200μl of LISS (Low Ionic Strength Solution).
Antibody screening or cross-matching using DTT or 2-ME treatment RBCs by IATs
Antibody screening cells and donor RBCs treated with DTT or 2-ME were used for antibody screening and cross-matching respectively by IATs method using Polyspecific Anti-Human Globulin Anti-IgG,-C3d Card (BIO-RAD, Cressier, Switzerland). Parallelly, E+ and K+ RBCs were treated with DTT or 2-ME and then reacted with anti-E and anti-K antiserum respectively synchronously as negative and positive controls to verify DTT or 2-ME treatment efficacy. If control K+ RBCs do not give negative reactions when tested with anti-K, the DTT/2-ME treatment has been inadequate; if control E+ RBCs do not give strong positive reactions when tested with anti-E, the DTT/2-ME treatment has been excessive.
Effect of 2-ME treatment on RBC blood group antigens
Anti-K, anti-D, anti-M, anti-N, anti-S, anti-s, anti-Jka, anti-Jkb, anti-Fya, anti-Fyb, anti-Dia IgG antibody reagents were purchased from CE-immundiagnostika GmbH, Germany. Anti-E, anti-e, anti-C, anti-c IgG antibodies were self-made reagents of human IgG antibodies in our laboratory. Anti-K, anti-D, anti-E, anti-e, anti-C, anti-c, anti-M, anti-N, anti-S, anti-s, anti-Jka, anti-Jkb, anti-Fya, anti-Fyb, anti-Dia antibody reagents were diluted with AB type plasma without irregular antibody to achieve titer 1 (polyspecific anti-human globulin anti-IgG, -C3d card (BIO-RAD, Cressier, Switzerland)). Antibody identification panel RBCs treated with 2-ME containing corresponding antigens were mixed with diluted corresponding antibody reagents to react in polyspecific anti-human globulin cards. The manufacture instructions were followed to observe whether 2-ME had a destructive effect on RBC antigens.
Antibody screening or cross-matching by Polybrene method
Since anti-E is the most common clinically significant RBC antibody in Chinese patients, human IgG anti-E was used as a positive control in Polybrene method. In order to verify the detectability of Polybrene method for clinically significant antibody with the same titer (by IATs) as DARA, anti-E antibody titers were standardized between plasma of DARA-treated patients and ABO compatible human IgG anti-E plasma by IATs using polyspecific anti-human globulin anti-IgG, -C3d card. Anti-E antibody was diluted by AB type plasma. The standardized anti-E antibody was selected as the control for subsequent antibody screening and cross-matching by Polybrene method. The Polybrene kit was purchased from SHPBC (Shanghai, China) and the manufacture instructions were followed: two drops of patient’s sera are placed in 12 × 75 mm glass tubes followed by the addition of one drop of donor’s RBCs or antibody screening cells; 600 µL of the low ion medium (LIM) is then added to facilitate rapid coating of the cells by antibodies. The mixtures are kept at room temperature in a low ionic phase for 1 min. Two drops of the working Polybrene solution is added to each tube and mixed. Fifteen seconds later, the tubes are centrifuged at 1000g for 10 s, and the cell-free supernatant fluids are decanted. Polybrene-induced agglutination could be observed when tubes are shaken and then the agglutination is reversed by adding two drops of the resuspending solution to the tubes, which are placed in a rack and then mixed gently. Hold the rack so that the tubes are kept at an angle of 45° allowing the cell suspensions to move more freely when the rack is shaken. Usually, within 10 s, the Polybrene-induced aggregates dissociate, and positive reactions can be readily observed to the naked eye. No agglutination within 1 min indicates negative result. Controls were performed simultaneously using diluted anti-E and E+ RBCs instead of patient’s plasma and donor RBCs in cross-matching test, and just using diluted anti-E instead of patient’s plasma in antibody screening test. The results were synchronously compared between patients’ plasma and the standardized anti-E plasma.
Results
Characteristics of routine pre-transfusion compatibility testing in Chinese patients undergoing DARA treatment
During the routine pre-transfusion detection of 24 Chinese patients treated with DARA, ABO, D, and other Rh phenotyping tests were not affected. Antibody screening ((A), left) or cross-matching ((B), left) was positive. The agglutination intensity was usually approximately 2+.
Figure 1. Representative results of pre-transfusion compatibility test using IATs in a Chinese patient undergoing DARA treatment. (A) Antibody screening results of patients before and after 2-ME/DTT treatment. (B) Cross-matching results of patients before and after 2-ME/DTT treatment. (C) Antigen detection results before and after 2-ME treatment in panel RBCs. -E, anti-E antibody; -K, anti-K antibody; S1 to S3, antibody screening cells 1–3; P, major cross-matching of this patient; -D, anti-D antibody; -C, anti-C antibody; -c, anti-c antibody; -e, anti-e antibody; -M, anti-M antibody; -N, anti-N antibody; -S, anti-S antibody; -s, anti-s antibody; -Jka, anti-Jka antibody; -Jkb, anti-Jkb antibody; -Fya, anti-Fya antibody; -Fyb, anti-Fyb antibody; -Dia, anti-Dia antibody.
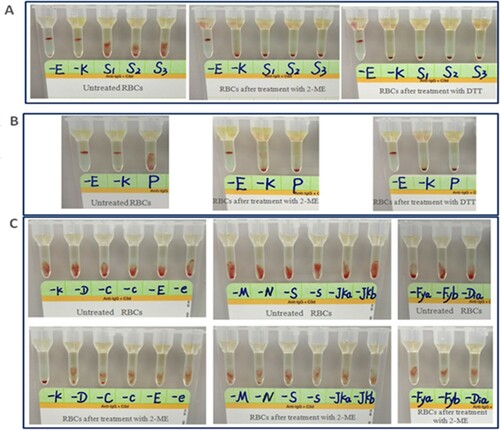
Antibody screening and cross-matching results using RBCs treated with 2-ME or DTT
When treating donor RBCs with DTT or 2-ME (Baso), no serious hemolysis was observed. While Serious hemolysis was observed when they were treated with 2-ME (SHPBC). The antibody screening tests based on the microcolumn gel method were performed on the antibody screening RBCs treated with 2-ME and plasma of MM patients after receiving DARA. The results were negative ((A), middle). When donors RBCs treated with 2-ME were cross-matched using a microcolumn gel method, panreactivity previously detected in the plasma of these patients by IATs was not observed ((B), middle). The efficacy of treatment was proved by the denaturation of the K antigen in the K+ control cell and preservation of the E antigen in the E+ control cell. The experimental results were consistent with those of DTT treatment of RBCs ((A), right; (B), right).
Effect of 2-ME treatment on RBC blood group antigens
As mentioned above, serious hemolysis was observed when reagent RBCs were treated with 2-ME (SHPBC) at 1:4 ratio, while no obvious hemolysis was seen until the end of incubation when reagent RBCs were treated with 2-ME (Baso) at 1:4 ratio. Agglutinations of the antibody identification panel cells with diluted corresponding antibodies before and after treated with 2-ME (Baso) were shown in (C). 2-ME had a destructive effect on K antigen, but not on D, E, e, C, c, M, N, S, s, Jka, Jkb, Fya, Fyb, Dia antigens.
Antibody screening and cross-matching tests of MM patients treated with DARA using Polybrene method
The agglutination intensity of human IgG anti-E plasma detected by gel card at different dilutions is shown in (A). For each sample, human IgG anti-E was standardized to a dilution of 128–256 to show the same agglutination intensity as DARA in IATs. Both of the antibody screening and cross-matching results of patients’ plasma using Polybrene method were negative, while both of the corresponding results of standardized IgG anti-E control were positive for approximate 2+ agglutination intensity ((B,C)).
Figure 2. Representative results of pre-transfusion compatibility test using Polybrene method in a Chinese patient treated with DARA. The results were all negative, while IgG anti-E plasma showed a 2+ agglutination intensity. (A) Different agglutination intensity of E+ RBCs reacted with human IgG Anti-E diluted from 1:16 to 1:256 in IATs. (B) Antibody screening results (left three tubes: anti-E control, right three tubes: Patient S1 to S3 means 3 antibody screening cells). (C) Major cross-matching results (left: anti-E control, right: Patient).
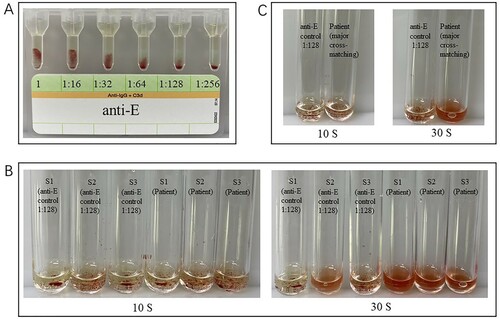
Discussion
As for mitigating DARA interference on the pre-transfusion compatibility test, it has been speculated that 2-ME may have a similar effect as DTT [Citation8]. Our study demonstrated that 2-ME can achieve the same treatment effect as DTT, though certain brand has been associated with severe hemolysis. The 2-ME reagent used in the experiment is a commercial product mainly used to destroy the IgM antibody in the serum. The product mixed with the serum 1:1 and added into the red blood cells will not cause hemolysis. The significant difference of hemolysis caused by different brands of reagents when the product mixed with 3% RBCs suspension 4:1 (V/V) may reflect the difference of diluents used by different manufacturers. Due to the high consistency of hemolysis degree caused by reagents from the same brand, the hemolysis mainly comes from 2-ME reagent rather than an irregular antibody. Therefore, the use of 2-ME from different manufacturers for treating RBCs to mitigate the interference of DARA therapy should be validated before use in diagnostics.
Thiol reductants can denature other important RBC antigens, such as Kell system antigens [Citation19]. In this study, we found that 2-ME destroys the Kell system but has no obvious destructive effect on other clinically relevant blood group antigens, which is similar to DTT. Kell system has polymorphism distribution in the Caucasian population with approximate 8-9% K+ phenotype frequency [Citation20]. Thus, K- units must be provided to these patients using DARA unless they are known to be K+. The East Asian populations usually carry a K-k+ type. The K+ blood group has a frequency of about 0.07%; thus, Kell blood grouping is not commonly performed [Citation21]. Consequently, Kell blood grouping after sulfhydryl reductant treatment of RBCs is not necessary for the Asian population, including the Chinese.
Similarly, the widespread use of the Polybrene method in pre-transfusion testing in the East Asian population is based on this consideration. Polybrene is a rapid and straightforward method for RBC antibody detection, which is used for pre-transfusion antibody detection and cross-matching test in many clinical laboratories across East Asian countries. The results of IATs after using DARA is positive, while that of Polybrene test is negative. At present, the use of Polybrene method to overcome the interference of DARA on pre-transfusion compatibility detection is scattered in few reports [Citation9,Citation17]. The results of 37 pre-transfusion compatibility tests using Polybrene method in this study were all negative, consistent with those of DTT treating methods. This demonstrated that Polybrene method mitigates the interference of DARA on blood compatibility tests.
We speculate that the underlying mechanism is similar to the Polybrene method's weak detection ability for Kell blood group antigens. Kell protein, like CD38 molecule, is a type II transmembrane glycoprotein with a single transmembrane, and a carboxyl group outside the cell. Polybrene is a small molecular weight quaternary ammonium that binds to negatively charged molecules on the surface of RBCs [Citation22]. Polybrene method has a weak detection ability for the Kell system's antibody, which is related to the negatively charged carboxyl group at the Kell protein terminal. The positively charged Polybrene molecule binding to the negatively charged Kell protein molecule in a low ionic medium environment interferes with the Kell system's antigen–antibody reaction. Based on the same mechanism, the Polybrene test is insensitive to CD38-related antigen–antibody reaction. Under a low ionic environment, the CD38 molecule carries a negatively charged carboxyl group extracellularly, which easily binds to the Polybrene molecule and interferes with the binding of the CD38 molecule to DARA.
In this study, standardized IgE antibodies which had the same titer as DARA in IAT medium were detected sensitively by Polybrene method, while DARA did not produce panreactivity in this method. This indicates Polybrene method could be a safe way to overcome the interference of Dara and detect red blood cell antibody at the same time. Since there is no other method to remove CD38 molecules from RBCs without destroying the Kell system's antigens, Polybrene method can also be used for pre-transfusion testing of Caucasian people under the premise of combining Kell system compatible transfusion. However, large-sample studies are needed to further verify these findings.
In conclusion, our data suggest that 2-ME treatment on RBCs may have a similar effect as DTT in patients with MM who received DARA treatment. Polybrene method is a simple and rapid procedure for the detection of red cell antibodies in the presence of DARA. 2-ME-based IATs or Polybrene method could be used to mitigate DARA interference on the premise of Kell system compatible transfusion. However, the use of different brand 2-ME should be validated before use in diagnostics to avoid a large amount of hemolysis.
Statement of ethics
The study was approved by the ethics committee of the participating institutions. Written informed consent was obtained from the patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- De Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–1848.
- Albeniz I, Demir O, Turker-Sener L, et al. Erythrocyte CD38 as a prognostic marker in cancer. Hematology. 2007;12:409–414.
- Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J. 1996;10:1408–1417.
- Zocchi E, Franco L, Guida L, et al. A single protein immunologically identified as CD38 displays NAD+ glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993;196:1459–1465.
- Oostendorp M, van Bueren L, Doshi JJ, et al. When blood transfusion medicine becomes complicated due to interference by monoclonal antibody therapy. Transfusion. 2015;55(6 Pt 2):1555–1562.
- Quach H, Benson S, Haysom H, et al. Considerations for pre-transfusion immunohaematology testing in patients receiving the anti-CD38 monoclonal antibody daratumumab for the treatment of multiple myeloma. Intern Med J. 2018;48:210–220.
- Chapuy CI, Nicholson RT, Aguad MD, et al. Resolving the daratumumab interference with blood compatibility testing. Transfusion. 2015;55(6 Pt 2):1545–1554.
- Chapuy CI, Aguad MD, Nicholson RT, et al. International validation of a dithiothreitol (DTT)-based method to resolve the daratumumab interference with blood compatibility testing. Transfusion. 2016;56(12):2964–2972.
- Yeh TJ, Yeh CJ, Liu YC, et al. Manual Polybrene method for pretransfusion test could overcome the interference of daratumumab therapy in myeloma. Transfusion. 2019;59:2751–2752.
- Berthelier V, Laboureau J, Boulla G, et al. Probing ligand-induced conformational changes of human CD38. Eur J Biochem. 2000;267(10):3056–3064.
- Dizon MF. The challenges of daratumumab in transfusion medicine. Lab Med. 2017;48(1):6–9.
- Selleng K, Gebicka PD, Thiele T. F(ab’)2 fragments to overcome daratumumab interference in transfusion tests. N Engl J Med. 2018;379(1):90–91.
- Werle E, Ziebart J, Wasmund E, et al. Daratumumab interference in pretransfusion testing is overcome by addition of daratumumab Fab fragments to patients’ plasma. Transfus Med Hemother. 2019;46(6):423–430.
- Lalezari P, Jiang AF. The manual Polybrene test: a simple and rapid procedure for detection of red cell antibodies. Transfusion. 1980;20:206–211.
- Ferrer Z, Wright J, Moore BP, et al. Comparison of a modified manual hexadimethrine bromide (Polybrene) and a low-ionic-strength solution antibody detection technique. Transfusion. 1985;25:145–148.
- Chu ML, Broadberry R. Experience with the manual Polybrene method in Taiwan. Transfusion. 1984;24:543.
- Xin Y, Yannan F, Chunya M, et al. Three cases of crossmatch incompatibility caused by daratumumab. Chinese Journal of Blood Transfusion. 2020;33:529–531. In Chinese.
- Fung MK, Eder A, Spitalnik SL, et al. AABB technical manual. 19th ed. Bethesda (MD): American Association of Blood Banks; 2017; method 3–18.
- Lancman G, Arinsburg S, Jhang J, et al. Blood transfusion management for patients treated with anti-CD38 monoclonal antibodies. Front Immunol. 2018;9:2616.
- Daniels G. Human blood groups. 3rd ed. Oxford: Wiley-Blackwell; 2013. p. 282–283.
- Lingling W, Ying Y, Ziyan Z. Molecular characteristics of Kell blood group K positive in Chinese population and development and application of typing kits. J Clin Transfus Lab Med. 2008;10:110–113. In Chinese.
- Klein HG, Anstee DJ. Immunology of red cells. Mollison’s blood transfusion in clinical medicine. 12th ed. London: Blackwell Science Ltd; 2013; p. 92.
