ABSTRACT
Introduction:
Invasive pulmonary aspergillosis is a life-threatening complication in the cases of patients with hematologic malignancies. In December 2019, the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and the Mycoses Study Group Education and Research Consortium published a revision and an update of the consensus definitions of invasive fungal disease. The aim of this study was to evaluate the signs and radiologic patterns of early-stage invasive pulmonary aspergillosis in computed tomography in patients with hematologic entities according to the latest criteria.
Material and methods:
This retrospective analysis of a baseline high-resolution computed tomography included neutropenic patients with hematological malignancies and probable invasive pulmonary aspergillosis. The data were collected between the years 2017 and 2019. Computed tomography was performed within 72 h from the beginning of clinical symptoms: fever, dyspnea or nonproductive cough. CT scans were analyzed by two independent radiologists according to the standardized protocol based on predefined criteria.
Results:
All 35 evaluated patients had typical lesions for early-stage invasive aspergillosis. Wedge-shaped infiltrates were noted in 48.6% of patients. In this group, 40% of patients had coexisting atypical radiological findings. In 11.4% of patients, wedge-shape consolidations were noted as the only type of lesions.
Conclusions:
Employment of the latest EORTC/MSG criteria increased diagnostic value of the baseline high resolution computed tomography in our study group by 11.4%.
Introduction
Invasive pulmonary aspergillosis (IPA) is the most prevalent mold infection. The frequency of IPA is significantly increasing in hematology patients. It is caused by excessive antibiotic therapy, employment of more intensive and immunosuppressive chemotherapy regimens and longer patient survival [Citation1]. IPA is a life-threatening and lethal condition unless promptly diagnosed and treated. One out of the two for early IPA diagnosis is computed tomography.
The incidence of invasive pulmonary aspergillosis is 8% in acute myelocytic leukemia (AML), 6.3% in acute lymphocytic leukemia (ALL), 12.8% in the cases of the patients following allogeneic hematopoietic stem-cell transplantation and 1.1% following autologous hematopoietic stem-cell transplantation [Citation2]. The major risk factors for developing IPA include neutropenia, prolonged therapy with high-dose corticosteroids, age over 65 and type of hematologic malignancy [Citation3]. In stem cell transplant recipients, other factors such as graft-versus-host disease play a role in the development of IPA [Citation4,Citation5]. Clinical symptoms of IPA are non-specific. Patients usually present with fever, dyspnea, cough and deterioration of clinical condition. Patients may also present with pleuritic chest pain and hemoptysis [Citation6]. Despite the use of mold-active prophylaxis since 1990s, invasive fungal infections remain a leading cause of morbidity in patients with hematological malignancies [Citation7]. The criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and the Mycoses Study Group Education and Research Consortium (EORTC/MSG) for diagnosing IPA are based on clinical features, host factors and microbiological evidence. Depending on which criteria are fulfilled, the diagnosis of an IPA is graded into possible, probable or proven categories. Proven IPA is when histopathologic, cytopathologic or direct microscopic examinations of specimens are positive and clinical, host criteria are met. Probable IPA is when clinical and host criteria are met and galactomannan antigen is detected in serum, plasma, bronchoalveolar lavage (BAL) or cerebrospinal fluid (CSF). Cases that meet the criteria for a host factor and a clinical criteria but mycological criteria are absent are considered possible [Citation8].
The clinical criteria include high-resolution computed tomography (HRCT) evaluation. In IPA, findings such as dense, well-circumscribed lesions with or without a halo sign, air crescent sign, cavity and wedge- shape, segmental or lobar consolidations may occur in HRCT (). Consolidations have been included to the criteria lately in the latest revision and an update of the EORTC/MSG criteria () [Citation8].
Figure 1. 66-year-old male with chronic lymphocytic leukemia, presenting with crepitations over the lung, cough and fever. High resolution CT scan obtained at level of pulmonary veins shows massive segmental consolidations with air bronchogram located in both lungs (black narrow arrows), nodules with halo sign (white narrow arrow) in lingular segment in left lung and free fluid in right pleural cavity (black wide arrow).
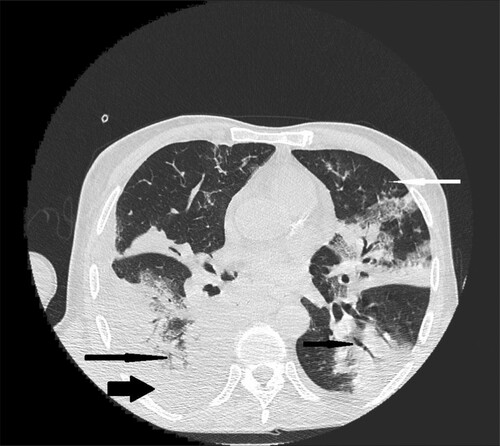
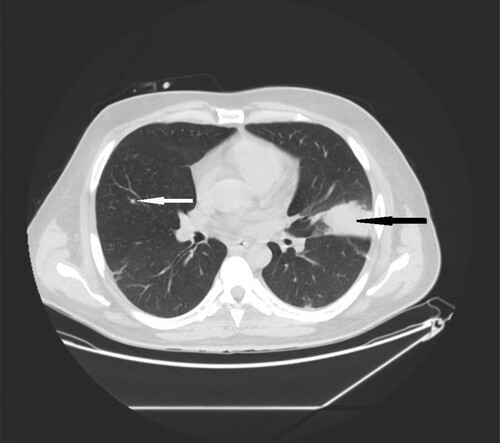
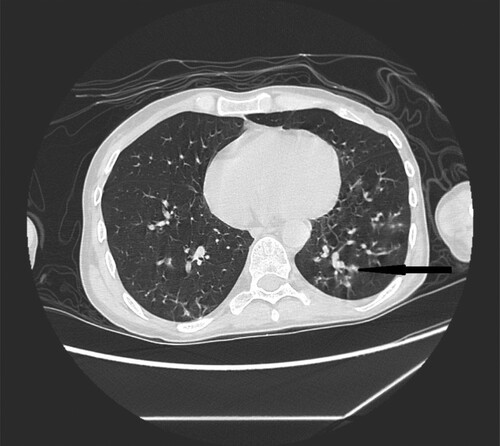
Figure 2. 35-year-old male with acute myeloid leukemia presenting with dry cough. High resolution CT obtained at level of pulmonary veins shows wedge-shape consolidations located in lingular segment in left lung (black narrow arrow). Nodule in the middle lobe of the right lung (white narrow arrow).
Early diagnosis and treatment are essential for improving patients’ outcome. Proven or probable IPA is associated with a low survival rate of 14% compared to 32% for patients without IPA [Citation9]. The diagnosis of early-stage IPA may be challenging. High resolution computed tomography (HRCT) is a rapid and useful tool for diagnosing IPA. In contrast to bronchoscopy or lung biopsy it is not invasive and easy to perform in patients with respiratory failure and comorbidities, such as severe thrombocytopenia or hypoxemia [Citation10,Citation11]. Accurate radiological evaluation is essential for the diagnosis of IPA and initiation of anti-fungal treatment.
The aim of this study was to evaluate if the application of updated radiological EORTC/MSG criteria increases the diagnostic value of HRCT.
Materials and methods
Study design and patient population
The data were collected retrospectively from January 2017 to January 2019. The period was chosen due to the availability of updated clinical and microbiological database system. To perform the study we used the following inclusion criteria: hematology patients presenting with clinical symptoms such as fever and lower respiratory tract infection, without any improvement after broad-spectrum antibacterial treatment. In all cases, a HRCT was performed within 72 h from the beginning of the symptoms. None of the patients had a previous history of an invasive mold disease. According to the revised EORTC/MSG criteria, all patients were diagnosed with probable invasive fungal disease, and were started on anti-mold treatment after the baseline HRCT. A follow-up HRCT was performed in all cases within 14 days from the diagnosis. None of the patients had positive bacterial blood or sputum cultures nor PCR tests for 15 respiratory viruses (including Influenza, Respiratory Syncytial Virus, Parainluenza, Metapneumovirus and others) or Pneumocystis jiroveci in bronchoalveolar lavage (BAL). Patients with negative galactomannan antigens from serum blood tests or BAL and with mixed etiology of pulmonary infection were excluded from the study. The study was performed according to the seventh revision of Declaration of Helsinki. The Institutional Review Board approved the study. Informed consent was not required due to the retrospective nature of the study.
HRCT scan evaluation
The HRCT scans were acquired using the GE 64-row scanner. The HRCT scans were obtained on supine position at the end-inspiratory effort. The thoracic scanning parameters were 120 kV, 240–460 mA, 1.25 mm collimation and a pitch of 0.984:1 (). From this dataset axial slices of 1 mm were obtained. The coronal and sagittal slices were as high resolution multiplanar reformation (MPR) images. All HRCT exams were obtained without intravenous contrast administration. Images were analyzed at picture archiving and communications systems (PACS).
Table 1. HRCT acquisition parameters.
The HRCT were reviewed twice and evaluation was made by two independent radiologists (one board specialist with more than 15-year experience in oncologic imaging and one senior resident) according to standardized protocol based on predefined criteria. The differences in interpretation were resolved by discussion or by the third board certified radiologist. All the clinical data required were accessible to radiologists.
For the study purposes, all pulmonary lesions were analyzed and grouped according to its morphology, size and location (). The lesions were divided into typical and atypical for IPA in line with the latest criteria. The criteria included typical lesions, such as nodules, nodules with halo, consolidations, air crescent signs and cavities (). The atypical findings were as follows: ground glass opacities (GGO), septal thickening, pleural effusions, enlarged lymph nodes.
Figure 3. 40-year-old female with cutaneous T-cell lymphoma presenting with fever and dyspnea. High resolution CT scans obtained at level of lower lobes shows consolidations (black narrow arrow) and multiple nodules with halo sign located in both lungs (white narrow arrow).
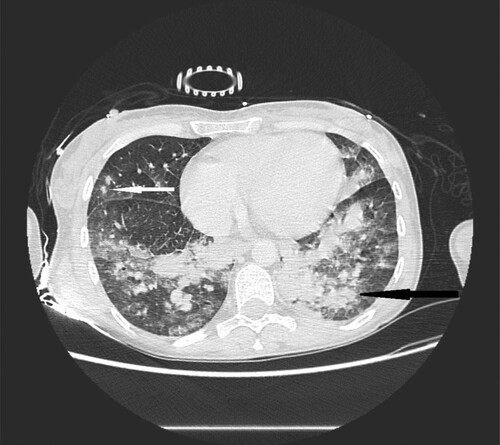
Table 2. Characteristics of pulmonary findings.
A standard glossary of terms for computed tomography was applied to describe the lesions [Citation12]:
nodule was defined as a well marginated round or ovoid shape soft tissue lesion, with the diameter up to 50 mm. Nodules smaller than 10 mm were considered to be micronodules and greater than 50 mm were considered as a consolidation ().
GGO was defined as hazy increased attenuation of lung with preserved bronchial and vascular margins.
nodule with halo sign was described as a nodule surrounded by a peripheral rim of GGO ( and ).
consolidation was considered to be an increase in pulmonary parenchymal attenuation that obscures the airways and vessels.
wedge- shape consolidations- were considered to be well, sharply marginated, with triangle shape in axial planes ( and ).
air crescent sign – was the crescentic collection of air surrounding the consolidation or nodule.
cavity- was a well-defined air space.
pleural effusion was described as a free fluid in the pleural cavity.
Other, non-infectious findings such as bronchial wall thickening, bronchiectasis and bullae, were also noted.
Figure 4. 72-year-old woman with acute myeloid leukemia presenting with fever. High- resolution CT scan obtained at level of main bronchi shows micronodules and nodules located peripherally in middle right lobe (black narrow arrow).
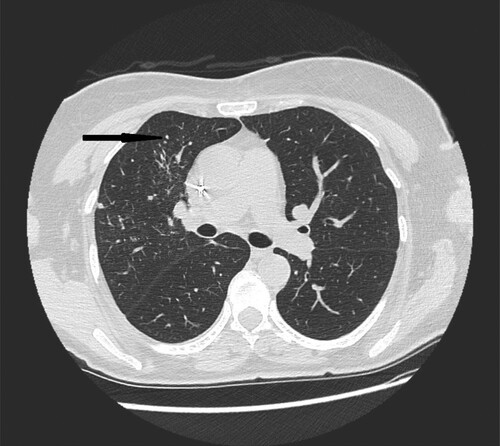
Figure 5. 62-year-old female with acute myeloid leukemia presenting with cough and fever. High resolution CT scan obtained at level of lower lobes shows nodules with halo sign located peripherally in right lower lobe (black narrow arrow).
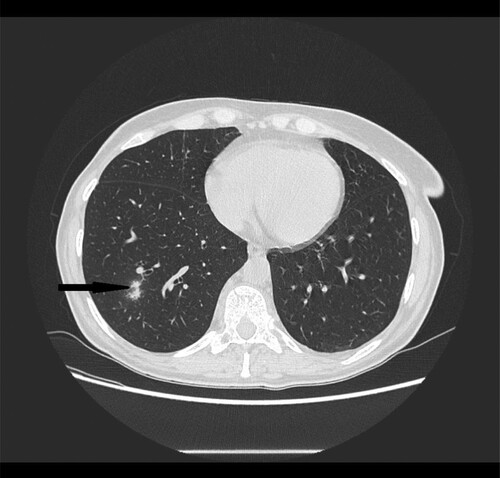
Figure 6. 56-year-old male with primary myeloid fibrosis 30 days after bone marrow transplantation presenting with fever. High resolution CT scan obtained at level of lower lobes shows peripherally located nodules with halo sign in right lower lobe (black narrow arrow).
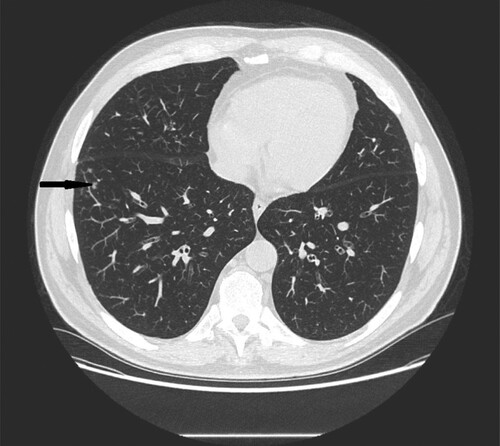
Figure 7. 28-year-old female with congenital neutropenia presenting with productive cough and fever. High resolution CT scans obtained at level of lower lobes shows massive consolidations with air- bronchogram located in basal segments of left lower lobe (black narrow arrow).
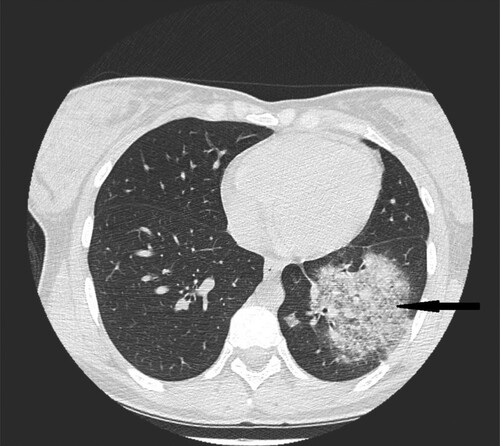
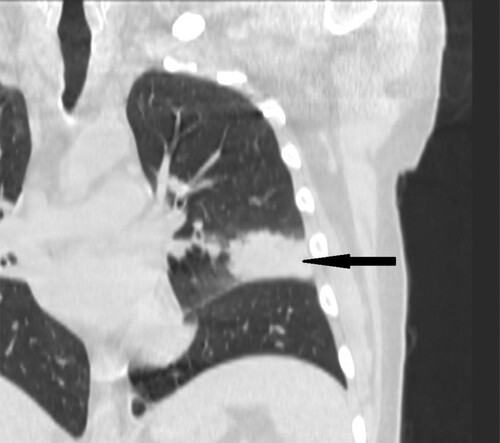
Figure 8. 35-year-old male with acute myeloid leukemia presenting with dry cough. High resolution CT in coronal plane shows wedge-shape consolidations located in lingular segment in left lung (black narrow arrow).
The distribution of lesions was considered to be central when located near the central, lobar or segmental bronchi or peripheral when surrounding the subsegmental bronchi ().
Figure 9. 37-year-old male with acute myeloid leukemia 26 days after bone marrow transplantation presenting with fever. High resolution CT scan obtained at level of lower lobes shows peripherally located nodules and micronodules with halo sign in left lower lobe (black narrow arrow).
The radiologists described the number and location of the lesions.
Microbiological analysis
Galactomannan is a highly immunogenic antigen therefore it has been recognized as a good marker of invasive aspergillosis. In soluble form, galactomannan antigen can circulate in body fluids at concentrations of 1–500 ng/ml.
In accordance with the guidelines of EORTC/MSG from 2002 to 2008, serological detection was performed once or twice weekly. Aspergillus spp. galactomannan antigen detection was performed using a sandwich immunocapture ELISA (Platelia- Aspergillus, Bio- Rad), according to the manufacturer’s recommendations in sera samples and/or bronchoalveolar lavage fluid (BALF) specimens. Results were expressed as an index of positivity. They were considered positive when ⩾0.5 either in sera samples or BALF.
Statistical analysis
The comparative data were analyzed by χ2 or Fisher’s exact test for categorical variables. For the location and size of lesions a chi-square was applied. A two-sided p-value of less than 0.05 was considered statistically significant. All statistical analyses were done with Statistica 13.3 package.
Results
The study included 35 patients, with a median of 65 (range 22–84), with hematological malignancies and neutropenia, hospitalized in the Hematology Department of the Medical University of Warsaw between January 2017 and January 2019.
Patients characteristics are presented in .
Table 3. Patients characteristics.
Invasive pulmonary aspergillosis was confirmed by positive galactomannan blood (n = 35) or BALF (n = 2) test.
All patients had abnormal findings in HRCT ().
Table 4. Characteristics of pulmonary findings.
In all patients typical findings of IPA occurred. In 11.4% wedge-shaped consolidations were seen without any other typical lesions.
In 21 patients (60%) we found coexisting atypical radiological lesions. GGO occurred in 31.4% and pleural effusion occurred in 34.3% of patients. Enlarged lymph nodes occurred in 11.4%. None of the patients had the atypical lesions alone.
The most common HRCT findings in our study were nodules with halo (88.5%) and wedge shape consolidations (48.6%). None of the patients presented any air crescent signs or cavities.
In patients with micronodules which are considered to be typical lesions, concomitant atypical findings were observed in 57.1% (p = 0.007) ().
Table 5. Location and number of lesions.
In patients with 10 or more lesions, the atypical pattern was dominant, and occurred in 48.6% (p = 0.02).
When lesions were located simultaneously centrally and peripherally, the atypical IPA features were coexisting in 62% of patients (p = 0.08).
In patients with 10 or more lesions, in 66.6% the distribution was both central and peripheral (p < 0.001) ().
Table 6. Location of the lesions according to the number of lesions.
Progression of the IPA was observed in the control HRCT in 10 patients (28.6%), the majority of this group (70%) consisted of patients with atypical IPA lesions. The early mortality rate, within three months after the baseline HRCT, was 25.7% and related to IPA progression. The overall mortality rate was 28.6%.
Discussion
The type of lesions considered typical for IPA has been expanding over time. Traditionally the most typical IPA findings were air-crescent signs and cavities [Citation13]. With the advent of diagnostic methods currently they are regarded as late signs. In our study group, they were not observed in any of the patients.
Currently, the majority of patients are diagnosed early in the course of IPA and typical HRCT findings include nodules, nodules with halo and consolidations [Citation14]. Interpretation of such findings must be always done in the context of patients’ clinical symptoms and results of laboratory tests. All patients in our study group presented with typical findings. The diagnostic procedures in hematology patients should be guided by the underlying disease, leukocyte count and clinical symptoms [Citation15].
According to the latest EORTC MSG criteria, the wedge shape, segmental or lobar consolidations were introduced as typical for IPA. This type of lesion occurred in 48.6% of our patients while 11.4% occurred as the only typical finding (see ). Segmental, lobar or wedge-shaped consolidations occur in bacterial pneumonia or pulmonary infarction due to pulmonary embolism. Other conditions that should be considered in differential diagnosis include septic pulmonary emboli and pulmonary hemorrhage [Citation16].
Nodules were found in 37.1% of patients (see ). As the differential diagnosis of pulmonary nodules is broad, the occurrence of relevant symptoms, the number of nodules, and their imaging characteristics (location, shape, presence and type of calcifications, and presence of spiculation or cavitation) may narrow the differential diagnosis. Even though considered typical for IPA their size and number should be verified with previous HRCT whenever possible. Nodules can be the manifestation of previous pneumonias [Citation17].
The halo sign is a well-known pattern of IPA [Citation18]. In our study group, it occurred in 88.5% (see ). The sign itself is non-specific. Apart from infection-related condition, many neoplastic and inflammatory processes can cause halo sign. However, in the context of patients with hematological malignancies, it suggests IPA [Citation19].
In the study group, 31.4% of patients had GGO (see ). However, when overlapping typical and atypical findings are seen, especially GGO, mixed pneumonia etiology should be considered. In such cases, differential diagnosis should include viral lower respiratory tract infections and Pneumocystis jiroveci [Citation20].
Pleural effusions were categorized as atypical findings. It occurred in 34.3% of patients, however, due to the older age of our study population and comorbidities the IPA etiology of pleural effusions is unlikely (see ). Overall, fungal infections account for less than 1% of all pleural effusions [Citation21].
Enlarged lymph nodes are a rare finding in IPA itself and this finding is reported in the literature occasionally, rather in case reports [Citation22,Citation23]. In our study group enlarged lymph nodes occurred in 11.4% (see ).
Another atypical finding for IPA is septal thickening. This finding may be the manifestation of different entities, corresponding with the Kerley B lines seen on X- rays [Citation24].
The major limitations of our study were retrospective data collection and a small study group. None of the patients in our study group had a histologically or culture proven IPA.
As the sensitivity of the serum testing is relatively low and radiologic signs of IPA have been shown to precede serum galactomannan testing [Citation25], the HRCT is a major diagnostic tool.
It suggests that in the cases of hematological malignancies, neutropenia and symptoms of infection, occurring with consolidations in baseline HRCT, the differential diagnosis should consider IPA.
Conclusions
Wedge-shape consolidations may add useful information for the diagnosis of invasive pulmonary aspergillosis in patients with underline disease of hematological malignancies. Employment of the latest EORTC/MSG criteria increased diagnostic value of the baseline high resolution computed tomography in our study group by 11.4%.
Data availability
The database used to support the findings of this study is available from the corresponding author upon request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Vazquez JA, Tovar-Torres MP, Hingwe A, et al. The changing epidemiology of invasive aspergillosis in the non-traditional host: risk factors and outcomes. Pulm Crit Care Med. 2016;1(3):67–71.
- Cornet M, Fleury L, Maslo C, et al. Epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the Greater Paris area. J Hosp Infect. 2002;51:288–296.
- Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139:519–531.
- Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011;49(Suppl 1):S7–S12.
- Marr KA, Carter RA, Boeckh M, et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100(13):4358–4366.
- Kousha M. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156–174.
- Young AY, LeivaJuarez MM, Evans SE. Fungal pneumonia in patients with hematologic malignancy and hematopoietic stem cell transplantation. Clin Chest Med. 2017;38(3):479–491.
- Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376.
- Michallet M, Bénet T, Sobh M, et al. Invasive aspergillosis: an important risk factor on the short- and long-term survival of acute myeloid leukemia (AML) patients. Eur J Clin Microbiol Infect Dis. 2012;31:991–997.
- Cattaneo C, Gramegna D, Malagola M, et al. Invasive pulmonary aspergillosis in acute leukemia: a still frequent condition with a negative impact on the overall treatment outcome. Leuk Lymphoma. 2019;60(12):3044–3050.
- Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60.
- Austin JH, Muller NL, Friedman PJ, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200:327–331.
- Brodoefel H, Vogel M, Hebart H, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. Am J Roentgenol. 2006;187(2):404–413.
- Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379.
- Bergeron A, Porcher R, Sulahian A, et al. The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood. 2012;119(8):1831–1837.
- He H, Stein MW, Zalta B, et al. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21:1–7.
- Loverdos K, Fotiadis A, Kontogianni C, et al. Lung nodules: a comprehensive review on current approach and management. Ann Thorac Med. 2019;14(4):226–238.
- Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–614.
- Lee YR, Choi YW, Lee KJ, et al. CT halo sign: the spectrum of pulmonary diseases. Brit J Radiol. 2005;78:862–865.
- Magira EE, Chemaly RF, Jiang Y, et al. Outcomes in invasive pulmonary aspergillosis infections by respiratory viral infections in patients with hematologic malignancies: a case control study. Open Forum Infect Dis. 2019;6(7):ofz247.
- Light RW. Pleural disease, Vol. 11. 4th ed. Baltimore: Williams and Wilkins; 2001; Pleural effusion secondary to fungal infections, actinomycosis, and nocardiosis. p. 196–203.
- Nourbakhsh E, Goodman S, Nugent K, et al. Generalized lymphadenopathy secondary to an invasive fungal infection in an apparently healthy patient. Am J Med Sci. 2010;340(1):84–88.
- Su SS, Zhou Y, Xu H-Y, et al. Invasive aspergillosis presenting as hilar masses with stenosis of bronchus: a case report. World J Clin Cases. 2019;7(22):3832–3837.
- Collins J. CT signs and patterns of lung disease. Radiol Clin North Am. 2001;39(6):1115–1135.
- Weisser M, Rausch C, Droll A, et al. Galactomannan does not precede major signs on a pulmonary computerised tomographic scan suggestive of invasive aspergillosis in patients with hematologic malignancies. Clin Infect Dis. 2005;41:1143–1149.
