ABSTRACT
Objectives
Chronic myeloid leukemia (CML) is a malignant tumor of the blood system. Gö6976, as a type of indolocarbazole and shows strong antitumor effects, but there have been no reports on the effect of Gö6976 on CML. The objectives of this research were: (1) to explore the impact of Gö6976 on CML in vitro and in vivo; and (2) to explore the drug toxicity of Gö6976 to normal cells and animals.
Methods
K562 cells and CML mice were used to explore the effect of Gö6976 on CML. Peripheral blood mononuclear cells (PBMCs), CD34+ cells, and healthy mice were used to explore the drug toxicity of Gö6976.
Results
Cell experiments showed that Gö6976 could inhibit the proliferation of K562 cells and enhance the inhibitory effects of imatinib at 5 μM and 10 μM, but it had little effect on CD34+ cells or PBMCs at concentrations less than 5 μM. Animal experiments showed that 2.5 mg/kg Gö6976 could effectively inhibit the development of CML in mice, and it had almost no effects on healthy mice at 2.5 mg/kg and 10 mg/kg.
Discussion
Because of the direct inhibitory effect of Gö6976 on CML and its pharmacological enhancement effect on imatinib, it is foreseeable that Gö6976 could become a new type of anti-CML medicine. And the further research is needed.
Conclusion
Our findings verified that Gö6976 could effectively inhibit CML in vitro and in vivo, and it is almost nontoxic to hematopoietic cells, immune cells, and healthy mice.
Introduction
As a malignant myeloproliferative tumor occurring in hematopoietic stem cells, CML is characterized by the Philadelphia chromosome, which is formed through reciprocal translocation between chromosomes 9 and 22 [Citation1,Citation2]. The BCR-ABL1 oncogene, which produces the chimeric BCR-ABL1 protein activating tyrosine kinase in leukemogenesis, will induce CML [Citation3]. With the development of tyrosine kinase inhibitors (TKIs) over the past 20 years, the outcomes of patients with CML have dramatically improved [Citation4,Citation5]. However, TKIs have some treatment-related complications, such as intolerance, failure of TKI therapy, or ineffective ‘dormant’ CML stem cells. Therefore, finding more effective targeted drugs for the treatment of CML remains a recent popular research topic.
Gö6976 is an indolocarbazole compound widely used as a specific inhibitor of PKCα/β [Citation6]. As a protein kinase C (PKC) inhibitor, it has shown remarkable effects in the treatment of collagen-induced arthritis [Citation7], melanoma [Citation8], nasopharyngeal carcinoma [Citation9] and lung cancer [Citation10]. Recently, some research has shown that Gö6976 can inhibit the development of acute myeloid leukemia (AML). In AML cells, Gö6976 can inhibit the phosphorylation of Fms-like tyrosine kinase 3 (FLT3) and downstream signaling molecules, such as STAT3/5, Erk1/2, and Akt, and FTL3, is key to the pathogenesis of AML [Citation11]. In primary AML cells, Gö6976 inhibited the activity of JAK2 and FLT3 tyrosine and reduced the activity of STAT, thereby inhibiting the proliferation of AML cells [Citation12]. However, research on Gö6976 in CML has not been reported, and there have been no systematic studies of its toxic effects in vivo.
Here, we investigated the effects of Gö6976 on K562 cells in vitro and K562-NOD/SCID CML mice in vivo. We also systematically explored the toxicity of Gö6976 to hematopoietic cells, immune cells and healthy mice.
Materials and methods
K562 cell lines and cells culture
K562 cells were established in our laboratory (Chongqing, China). Cells were grown in RPMI 1640 medium (Gibco, Grand Island, USA) containing 10% fetal bovine serum (Gibco, Australia), 100 µg/mL streptomycin, and 100 U/mL penicillin (Beyotime, Shanghai, China). All of the cells were cultured and maintained in a 37 °C incubator with 5% CO2 and saturated humidity. The medium or subculture cells were changed every 24 h, and cells grown in logarithmic phase were collected for experimental studies.
Separation of PBMCs by the Ficoll-Paque method
Maternal umbilical cord blood samples (Chongqing, China) were collected and placed in an anticoagulant tube containing EDTA anticoagulant (BD, Shanghai, China). Two milliliters of Hank’s solution (Solarbio, Beijing, China) was added to the specimen, 2 mL of Ficoll solution (Solarbio, Beijing, China) was added to the mixture, and the mixture was centrifuged at 2000rpm for 20 min. The mononuclear cells in the white membrane layer were aspirated and transferred to 2 mL of Hank’s solution and then centrifuged at 1000 rpm for 10 min. The supernatant was discarded, and the cells were washed once again with Hank’s solution. Then, we transferred the obtained PBMCs to stem cell culture medium containing SCF (50 ng/mL), IL-6 (10 ng/mL) and IL-3 (10 ng/mL) and placed them in a cell incubator for subsequent experiments.
Sorting CD34+ cells by magnetic bead-based methods
The separated PBMCs were washed once with PBS (Gibco, Shanghai, China) and centrifuged at 2000rpm for 10 min. We discarded the supernatant, resuspend the cell pellet in 300 μL of PBS, added 100 μL of blocking solution (NCM, Suzhou, China) and 100 μL of CD34+ immunomagnetic beads (Thermo Fisher, Waltham, USA), mixed them well, incubated them in a 2–8 °C refrigerator for 30 min, washed the cells with 5-10 mL of PBS and centrifuged them at 1500 rpm for 10 min. The supernatant was discarded, and the cells were washed once with Hank’s solution, and then the supernatant was discarded again, and the cells were resuspended in 500 μL of PBS and placed on ice. The cell suspension was added to the MS separation column, and the centrifuge tube was washed 3 times with PBS. The separation column was removed from the MACS magnetic field, 1 mL of PBS was added, and all of the liquid was drained from the column into a centrifuge tube and centrifuged at 1500 rpm for 10 min.
PBMCs and CD34+ cells culture
The isolated PBMCs and CD34+ cells in stem cell culture medium containing SCF (50 ng/mL), IL-6 (10 ng/mL) and IL-3 (10 ng/mL) were separated. The medium was placed in a cell incubator at 37 °C, 5% CO2, and saturated humidity. Then, the medium was changed or passed on every 24 h, and the cells in the logarithmic growth phase were obtained for subsequent experimental research.
Cells counting kit-8 assay
K562 cells in logarithmic growth phase were collected and seeded into 96-well cell culture plates at a density of 5×103 cells/well. K562 cells were treated with 5 μM Gö6976, 10 µM Gö6976, 0 μM Gö6976 + 2 μM imatinib, 5 μM Gö6976 + 2 μM imatinib, and 10 μΜ Gö6976 + 2 μM imatinib, and the control group was only treated with PBS. Five parallel wells were set in each group and cultured for 72 h. Ten microliters of Cell Counting Kit-8 (CCK-8) solution (Solarbio, Shanghai, China) was added to each well. After incubation for 3 h at 37 °C in a 5% CO2 incubator, the absorbance at 450 nm was recorded.
MTT assay
CD34+ cells and PBMCs in logarithmic growth phase were collected and seeded in 96-well cell culture plates at a density of 5×103 cells/well. CD34+ cells and PBMCs were treated with 0.5, 1.0, 2.5, and 5.0 μM Gö6976 for 24 h. Five parallel wells were set in each group and cultured for 72 h. Then, 50 μL of 2 mg/mL MTT (Solarbio, Shanghai, China) solution were added to each well, and the cells were cultured for 4 h and centrifuged at 2000 r/min for 10 min. The supernatant was discarded in the dark. Finally, 100 μL of DMSO were added to each well, the mixture was shaken for 5 min, and the absorbance of each well was read at 450 nm.
Mice
NOD/SCID mice and BALB/c mice at 6 weeks of age, 19.65 ± 1.95 g in weight, SPF grade, were purchased and raised at the Experimental Animal Center of Chongqing Medical University (Chongqing, China). All of the mice were maintained in a temperature- and humidity-controlled environment and given acidified water. All of the experimental procedures were performed according to animal care and ethics legislation, and the research was approved by the Animal Care Committee of Chongqing Medical University.
Induction of CML in mice
To establish K562-NOD/SCID CML mice, 6- to 8-week-old NOD/SCID mice were injected with K562 cells. Recipient NOD/SCID mice lethally irradiated with 300 cGy were intravenously injected with approximately 5×106 K562 cells. The general condition of the mice was observed every day, peripheral blood of the mice was collected every week, and the number of White blood cells (WBCs) and the proportion of CD45+ cells were examined.
In vivo drug treatment
Drug treatments began at 8 days after K562 cell injection and were administered as two doses per week for 4 weeks. According to whether it was administered with Gö6976 (Mce, New Jersey, USA), K562-NOD/SCID CML mice were divided into two groups: mice in the experimental group injected with 2.5 mg/kg Gö6976 and mice in the control group injected with the same volume of normal saline.
In vivo drug toxicity experiment
Twenty-four BALB/c mice were randomly divided into 3 groups: mice in the experimental group injected with 2.5 mg/kg Gö6976 or 10 mg/kg Gö6976 and mice in the control group injected the same volume of normal saline. The mice were weighed and injected 12 times every 2 days. After the injections were stopped, blood and organs from these mice were collected.
Flow cytometric analysis
Cells were collected from the peripheral blood of mice. Red blood cells (RBCs) were lysed with red blood cell lysis buffer. Then, the remaining cells were washed with PBS and incubated with CD45-FITC (Thermo Fisher, Waltham, USA). Subsequently, the cells were treated with permeabilization wash buffer and stained. After washing, the cells were subjected to flow cytometric analysis.
White blood cells count
WBCs in peripheral blood from the CML-Gö6976 group and the CML-control group were counted once per week. We cut the tails of the mice to obtain peripheral blood. Then, 20 μL of blood were added to 380 μL of WBC dilution and gently mixed. After the RBCs were lysed, diluted blood was added to the blood cell counting pool. After 2-3 min, WBCs were counted under a microscope.
Hematoxylin–eosin staining
The livers, spleens and lungs of mice were separated and fixed in 4% (w/v) paraformaldehyde. Appropriately sized tissues (1.0 cm × 1.0 cm × 0.5 cm) were cut out and made into paraffin sections (4 μm thick). Then, the paraffin sections were dewaxed in xylene, rehydrated in a descending alcohol series, and stained with hematoxylin and eosin. The cell smears were air dried and stained with Wright’s staining solution A for 30 s and then solution B for 45 s. Finally, the sections were rinsed with tap water and observed under a microscope.
Statistics
Statistical analysis was performed using IBM SPSS statistical software (version 26.0, Chicago, USA). The results are presented as the means ± the standard error and were analyzed by variance. General observations and histopathological findings are descriptively summarized. Statistical significance was defined as P < 0.05.
Results
Gö6976 inhibited the proliferation of K562 cells and resulted in no significant inhibition of normal cells
First, we conducted in vitro experiments to explore the effect of Gö6976 on leukemia cells. The CCK-8 assay results showed that the optical density (OD) of K562 cells in the Gö6976 groups was decreased at 24, 48 and 72 h compared with that in the control group. However, the OD in only the 10 μM Gö6976 group was significantly decreased compared with that in the control group, and the OD in the 5 μM Gö6976 group was not significantly different. At the same time, compared with that in the 5 μM Gö6976 group, the OD in the 10 μM group was decreased significantly after 24 h. ((a)).
Figure 1. The effect of Gö6976 on K562 cells and normal cells. (a) CCK-8 assay to analyze the effect of Gö6976 on the proliferation of K562 cells (*P < 0.01 versus the control group). (b) CCK-8 assays to analyze the effect of imatinib combined with Gö6976 on K562 cells (*P < 0.01 versus the control group or the 2μM imatinib group). (c) MTT assay to analyze the effect of Gö6976 on the proliferation of CD34+ cells and PBMCs.
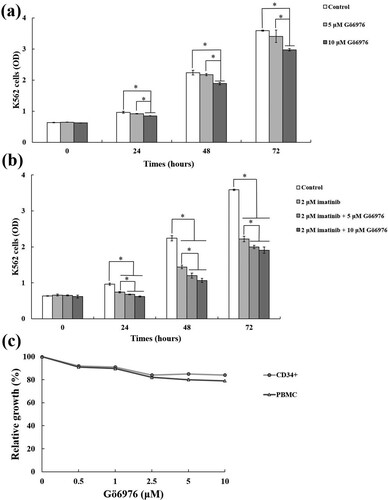
To further explore whether Gö6976 affects the anti-CML effect of TKIs, we added imatinib for the study. We found that the proliferation of K562 cells in the treatment groups was significantly inhibited compared with that in the control group after 24 h. At the same time, the proliferation of K562 cells in the groups treated with imatinib and Gö6976 was more inhibited than in the group treated with imatinib alone, and the difference was statistically significant. The OD of K562 cells in the imatinib combined with 10 μM Gö6976 group was reduced compared with that in the imatinib combined with 5 μM Gö6976 group, but the difference was not statistically significant ((b)).
The above results indicate that Gö6976 has a significant inhibitory effect on the growth of K562 cells and can enhance the sensitivity of K562 cells to imatinib. Then, we tested the effect of Gö6976 on hematopoietic cells and immune cells. The MTT assay results showed that there was no obvious inhibitory effect on CD34+ cells and PBMCs after treatment with 0.5, 1.0, 2.5 μM or 5.0 μM Gö6976 for 24 h. The relative growth rates were still approximately 80% ((c)).
Gö6976 can effectively inhibit the progression of CML in mice
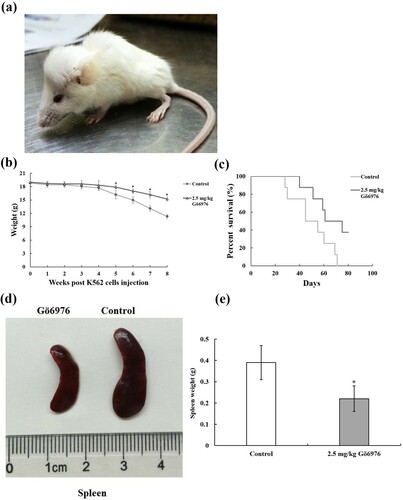
To explore the effects of Gö6976 in the body, we created a mouse model of CML. Mice began to develop leukemic symptoms at approximately 3 weeks after injection of tumor cells, including hindlimb paralysis, weight loss, fluffy hair and reduced activity ((a)). Along with the experimental data that follow, the K562-NOD/SCID CML mouse model was successfully established. After K562 cells were injected, CML mice were weighed and recorded weekly. Weight loss of the mice was found in both the Gö6976 group (2.5 mg/kg) and the control group. Compared with the control group, the mice in the Gö6976 group had significantly less weight loss 5 weeks after injection ((b)).
Figure 2. The therapeutic effect of Gö6976 on CML mice. (a) K562-NOD/SCID CML mice showed hindlimb paralysis, weight loss, fluffy hair and reduced activity. (b) Weight of mice at different time after K562 cells injection (*P < 0.05 versus control group). (c) Survival curves of two group mice (8 mice per group). (d) General morphology of spleens from two group mice. (e) Weight of Spleens from two group mice.
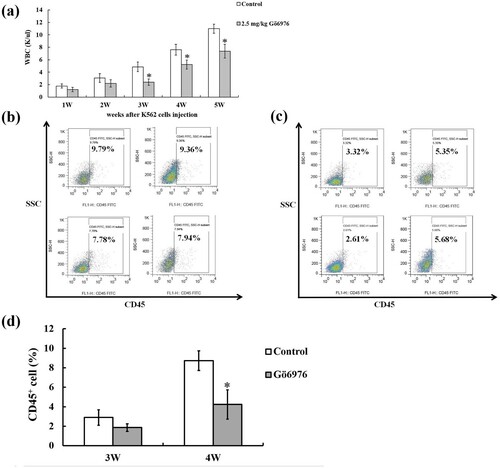
We collected the peripheral blood of the mice during the experiment and found that WBCs showed an upward trend in both groups. However, the number of WBCs in the 2.5 mg/kg Gö6976 group was less than that in the control group beginning the first week after injection. The number of WBCs in the 2.5 mg/kg Gö6976 group was significantly decreased compared with that in the control group from the 3rd to the 5th weeks ((a)). At the same time, the percentage of CD45+ leukemia cells in the peripheral blood of mice at different times was observed by flow cytometry. The results showed that the proportion of CD45+ leukemia cells in the WBCs from peripheral blood was only approximately 1% at the first and second weeks, and it was approximately 2-3% at the third week. There was no significant difference between the two groups in the first to third weeks. At the fourth week, the CD45+ leukemia cells in the 2.5 mg/kg Gö6976 group were significantly fewer than those in the control group ((b–d)).
Figure 3. Peripheral blood analysis of two group of mice, including white blood cells count and flow cytometry analysis of the percentage of CD45+ leukemia cells. (a) WBCs count in peripheral blood from the control group and the Gö6976 group (*P < 0.05 versus the control group). (b) The percentage of CD45+ leukemia cells in the control group on week 4. (c) The percentage of CD45+ leukemia cells in the Gö6976 group on week 4. (d) The percentage of CD45+ leukemia cells in peripheral blood from two groups on week 3 and week 4 (*P < 0.05 versus the control group).
The death time of the two groups of mice was recorded. The first mouse died in the control group on the 29th day after injection, and the last mouse in the control group died on the 71st day after transplantation. In the Gö6976 group, the first mouse died on the 40th day after injection, and three mice survived until the 80th day of the observation period. The survival time of the mice in the 2.5 mg/kg Gö6976 group was significantly longer than that of the mice in the control group. ((c)). Finally, the spleens were collected and weighed at the 9th week after injection. We found that the spleens of mice from the 2.5 mg/kg Gö6976 group were smaller than those from the control group ((d)), and the weights of the spleens were also lower than those from the control group ((e)).
Gö6976 has no significant effects on the general condition, liver, spleen or lungs of healthy mice
Through the above experiments, we proved that Gö6976 had a significant inhibitory effect on CML in vivo and in vitro. We found that Gö6976 had almost no effect on ordinary hematopoietic cells and immune cells in the in vitro experiments ((c)). Thus, whether it has an effect on healthy mice will be our next research direction.
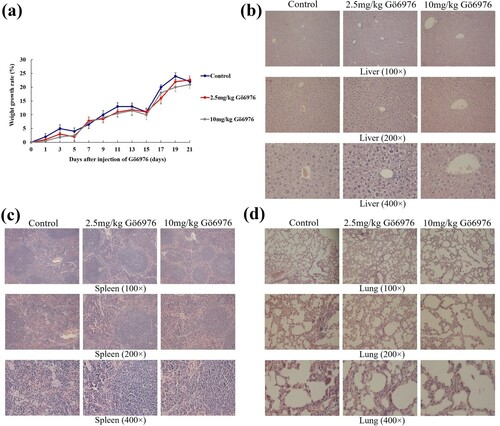
We divided healthy BALB/c mice into three groups: the control group (normal saline), the low-dose Gö6976 group (2.5 mg/kg Gö6976) and the high-dose Gö6976 group (10 mg/kg Gö6976). We found that there were no abnormalities in mental state, daily activities, food intake, or bowel movements of the mice in each group during the administration, and there was no significant difference in weight gain among the control group, the low-dose Gö6976 group and the high-dose Gö6976 group ((a)). The growth trend of these three groups remained consistent. The livers, spleens, and lungs were obtained and stained with HE at 3 weeks after injection. The tissue structure of these organs from the three groups was observed under light microscopy, and there was no inflammatory infiltration, cell degeneration, necrosis, interstitial hyperemia, edema or other pathological reactions in these two Gö6976 groups compared with the control group ((b–d)).
Figure 4. The effect of Gö6976 on the weight and organs of healthy mice. (a) The weight growth rate of the mice in each group. (b) Liver sections of the mice in each group after HE staining. (c) Spleen sections of the mice in each group after HE staining. (d) Lung sections of the mice in each group after HE staining.
Discussion
CML is a malignant tumor that threatens human life, and the BCR/ABL1 fusion gene is an important factor in the development and deterioration of CML [Citation13]. Recently, increasing evidence has shown that TKIs, a common anti-CML medicine, have little effect on ‘dormant’ CML stem cells [Citation14]. However, ‘dormant’ CML stem cells are the key cause of CML recurrence and refractoriness. Therefore, it is important to find a new anti-CML medicine. As a type of indolocarbazole, Gö6976 can effectively inhibit the activity of PKC, thereby exerting anti-inflammatory, anti-immune and antitumor effects [Citation15]. Gö6976 can inhibit tumor cell proliferation, slow tumor angiogenesis, and prevent tumor metastasis [Citation16,Citation17]. Gö6976 can act on many diseases, such as collagen-induced arthritis, melanoma, nasopharyngeal carcinoma and lung cancer [Citation7–10]. However, the role of Gö6976 in CML remains unknown, and the toxicity of Gö6976 in mice has not yet been reported. Therefore, we explored the role of Gö6976 in CML and the toxic effects of Gö6976 in in vivo and in vitro experiments.
In this study, we demonstrated that Gö6976 could significantly inhibit the proliferation of K562 cells at a concentration of 10 μM. Although the proliferation of K562 cells was also inhibited in the 5 μM group, the difference was not statistically significant. Furthermore, Gö6976 increased the sensitivity of K562 cells to imatinib at 5 µM and 10 μM. Therefore, it can be predicted that Gö6976 could be used as a sensitizer to enhance the sensitivity to TKIs of CML at low concentrations, and it could also be used alone at high concentrations to suppress drug-resistant CML.
To further verify our conclusions, we repeated them in animal experiments. After establishing K562-NOD/SCID CML mice, we injected 2.5 mg/kg Gö6976 into the experimental group and injected the same amount of normal saline into the control group. Compared with the control group, the weight loss of mice in the Gö6976 group was significantly slowed, the WBCs were significantly decreased, the percentage of CD45+ leukemia cells in the peripheral blood were significantly smaller, and the survival time was prolonged. This evidence showed that, after the injection of Gö6976 in CML mice, Gö6976 could effectively inhibit the development of CML in mice. Then, we dissected the mice and found that the volume and weight of the spleens of the mice in the Gö6976 group were significantly smaller than those in the control group, showing that the infiltration of the spleen by leukemia cells in mice was reduced by Gö6976. The therapeutic effect of Gö6976 in CML mice is obvious, and it can effectively relieve the clinical symptoms and organ damage of CML mice.
Finally, we conducted experiments to explore the effects of Gö6976 on hematopoietic cells, immune cells, and healthy mice. We treated CD34+ cells and PBMCs with different doses of Gö6976. Gö6976 had very weak effects on CD34+ cells and PBMCs. The detection of CD34+ cells and PBMCs could reflect the influence on hematopoietic function and immune cells, so this result could prove that Gö6976 has almost no influence on normal cells in vitro. After injection of Gö6976 into healthy mice, there were no significant changes in the general condition of the mice and no significant changes in the weights of the mice, and no significant organ damage was found in the tissue sections. All of these results demonstrated that there were no side effects on mice treated with Gö6976 in vivo.
In summary, Gö6976 can directly inhibit the progression of CML, effectively increase the survival rate of mice, and enhance the effect of imatinib in CML. At the same time, Gö6976 has almost no side effects on normal cells and organs.
Acknowledgments
This work was supported by the [Science and Technology Research Program of Chongqing Municipal Education Commission] under Grant [No. KJ1600225]. This research was conducted under the guidance of Professor Zhimin Lu from Zhejiang Provincial Key Laboratory of Pancreatic Disease, China. Prof. Lu owns Gö6976 related patents and has given us tremendous technical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gong Z, Wang W, Hu S. Cytogenetic alterations in CML: not all created equal. Oncotarget. 2018;9:11885–11886. doi:https://doi.org/10.18632/oncotarget.24471.
- Houshmand M, Simonetti G, Circosta P, et al. Chronic myeloid leukemia stem cells. Leukemia. 2019;33:1543–1556. doi:https://doi.org/10.1038/s41375-019-0490-0.
- Jain S, Abraham A. BCR-ABL1-like B-acute lymphoblastic leukemia/lymphoma: A comprehensive review. Arch Pathol Lab Med. 2020;144:150–155. doi:https://doi.org/10.5858/arpa.2019-0194-RA.
- Valent P, Hadzijusufovic E, Hoermann G, et al. Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res. 2017;59:47–54. doi:https://doi.org/10.1016/j.leukres.2017.05.008.
- Copland M. Is there a role for dose modification of TKI therapy in CML? Curr Hematol Malig Rep. 2019;14:337–345. doi:https://doi.org/10.1007/s11899-019-00524-w.
- Jung SY, Kim OB, Kang HK, et al. Protein kinase Cα/β inhibitor Gö6976 promotes PC12 cell adhesion and spreading through membrane recruitment and activation of protein kinase Cδ. Exp Cell Res. 2013;319:153–160. doi:https://doi.org/10.1016/j.yexcr.2012.10.003.
- Yoon TW, Kim YI, Cho H, et al. Ameliorating effects of Gö6976, a pharmacological agent that inhibits protein kinase D, on collagen-induced arthritis. PloS One. 2019;14:e0226145.
- Merzoug-Larabi M, Spasojevic C, Eymard M, et al. Protein kinase C inhibitor Gö6976 but not Gö6983 induces the reversion of E- to N-cadherin switch and metastatic phenotype in melanoma: identification of the role of protein kinase D1. BMC Cancer. 2017;17:12. doi:https://doi.org/10.1186/s12885-016-3007-5.
- Feng Z, Xu S, Liu M, et al. Chk1 inhibitor Gö6976 enhances the sensitivity of nasopharyngeal carcinoma cells to radiotherapy and chemotherapy in vitro and in vivo. Cancer Lett. 2010;297:190–197. doi:https://doi.org/10.1016/j.canlet.2010.05.011.
- Yao J, Zhao X, Ding X. Systematic profiling of chemotherapeutic drug response to EGFR gatekeeper mutation in non-small cell lung cancer. Comput Biol Chem. 2016;64:126–133. doi:https://doi.org/10.1016/j.compbiolchem.2016.05.009.
- Yoshida A, Ookura M, Zokumasu K, et al. Gö6976, a FLT3 kinase inhibitor, exerts potent cytotoxic activity against acute leukemia via inhibition of survivin and MCL-1. Biochem Pharmacol. 2014;90:16–24. doi:https://doi.org/10.1016/j.bcp.2014.04.002.
- Grandage VL, Everington T, Linch DC, et al. Gö6976 is a potent inhibitor of the JAK 2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br J Haematol. 2006;135:303–316. doi:https://doi.org/10.1111/j.1365-2141.2006.06291.x.
- Gupta P, Zhang GN, Barbuti AM, et al. Preclinical development of a novel BCR-ABL T315I inhibitor against chronic myeloid leukemia. Cancer Lett. 2020;472:132–141. doi:https://doi.org/10.1016/j.canlet.2019.11.040.
- Yaghmaie M, Yeung CC. Molecular mechanisms of resistance to tyrosine kinase inhibitors. Curr Hematol Malig Rep. 2019;14:395–404. doi:https://doi.org/10.1007/s11899-019-00543-7.
- Martiny-Baron G, Kazanietz MG, Mischak H, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268:9194–9197.
- Jia XZ, Yang SY, Zhou J, et al. Inhibition of CHK1 kinase by Gö6976 converts 8-chloro-adenosine-induced G2/M arrest into S arrest in human myelocytic leukemia K562 cells. Biochem Pharmacol. 2009;77:770–780. doi:https://doi.org/10.1016/j.bcp.2008.11.008.
- Bokhari SM, Zhou L, Karasek MA, et al. Regulation of skin microvasculature angiogenesis, cell migration, and permeability by a specific inhibitor of PKCalpha. J Invest Dermatol. 2006;126:460–467. doi:https://doi.org/10.1038/sj.jid.5700071.
