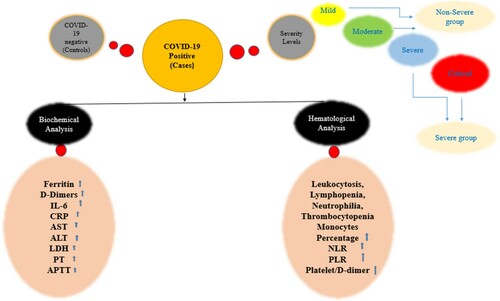
ABSTRACT
Objectives: This study was conducted to investigate alteration in blood parameters and their association with the presence, severity, and mortality of COVID-19 patients as the data on hematological abnormalities associated with the Pakistani COVID-19 patients is limited.
Methodology: A double-centered, hospital-based comparative retrospective case study was conducted, to include all the admitted patients (n = 317) having COVID-19 Polymerase chain reaction (PCR) positive. The control group (n = 157) tested negative for COVID-19.
Results: Of 317 admitted cases, the majority were males n = 198 (62.5%). Associated comorbidities, lower lymphocytes, platelets, and higher White blood cells, neutrophil, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were found in COVID-19 cases as compared to healthy controls (p < 0.001 for all). The biochemical parameters of cases including Ferritin, D-Dimer, CRP, IL-6, LDH, ALT, AST, and APTT also showed a statistically significant difference compared with standard values (p < 0.001 for all). However, their comparison with a severity level of the severe and non-severe groups showed significance for WBCs, neutrophils, NLR (p < 0.001 for all), and PLR (p = 0.06) only. Receiver operating characteristic curve analysis showed that NLR had the highest area under curve (0.84) followed by 1/lymphocyte (0.82), neutrophils (0.74), PLR (0.67),1/platelets (0.68) and WBC's (0.65). Comparison of cases and controls with recommended cut-off values derived from sensitivity and 1-specificity was also done (p < 0.001).
Conclusion: Monitoring all the hematological and biochemical parameters including novel hemograms NLR, PLR can aid clinicians to identify potentially severe cases at early stages and initiate effective management in time which may reduce the overall mortality of COVID-19 patients.
What is already known about this subject?
Hematological and biochemical parameters can serve as prognostic markers as they show changes with disease severity depicted in different studies.
While they can be a source of diagnosing COVID-19 infections, not all patients show the same changes. Different studies show the difference in their mean values, with values becoming more dispersed as the disease worsened and also in the deceased patients.
In other countries, changes show lymphopenia, leukocytosis, eosinopenia, and neutrophilia in severely affected patients, while changes in platelet count were differently reported in different studies.
Elevated D-dimers, fibrin degradation products, PT, APTT, ferritin, CRP, NLR, PLR, were reported in the literature, with the values increasing as the disease worsens.
What are the new findings?
In the Sahiwal division, an underdeveloped division of Pakistan, this is the first study of its kind reporting changes in hematological and biochemical parameters of patients and disease severity.
The data set of Comorbidities () gives valuable information for assessing mortality and severity in patients with comorbid conditions and their age, gender, race, quality of healthcare delivered to them, and socioeconomic conditions.
Healthy controls were compared with cases for CBC parameters. Further, biochemical parameters were compared with normal values, and disease severity was also determined between cases and controls.
An optimal cutoff value was recommended for statistically significant parameters.
How might it impact clinical practice in the foreseeable future:
Potential changes in patient's complete blood counts and inflammatory and biochemical markers can serve as a potential diagnostic tool as they are accessible and readily available and they can replace rRT-PCR in those countries which suffer from a significant short supply of rRT-PCR reagents.
CBCs can serve as a very significant laboratory examination. Since, their level changes after the onset of disease, they can truly identify the stage of the disease. Additionally, they are a key indicator to provide information for the diagnosis and treatment basis for health professionals.
1. Introduction
The start of 2020 has become a horrifying and unforgettable memory because of a novel virus, COVID-19, which abolished the world's normal lifestyle and health condition [Citation1]. The virus responsible for the pandemic is the SARS-CoV-2 RNA virus (severe acute respiratory syndrome coronavirus 2). The virus goes to pulmonary epithelial cells via surface ACE2 receptors, causing viral pneumonia, followed by a systemic inflammation phase which further goes on to an advanced stage to develop respiratory failure and multi-organ dysfunction. To control the rapid transmission of the COVID-19 pandemic, diagnostic tools would be of great value to detect cases accurately [Citation2]. Different molecular techniques were developed, but the healthcare professionals in developing countries have limited access to these specialized instruments. Limitations of real-time reverse transcriptase-PCR include a long turnaround time, need for expensive equipment, balancing cost on a large scale, and trained staff, as well as 20% false-negative results [Citation3–5]. Hence, there is a need for readily available tests for large-scale screening to rapidly diagnose the cases and prevent the overwhelming spread of this pandemic [Citation6].
Different biochemical and hemocytometric markers have been used in routine practice as changes in these markers have been reported in various studies and could serve as prognostic markers in disease severity. Moreover, patients having hematological abnormalities are at increased risk of having different infections and other comorbidities [Citation7]. According to international guidelines, hemocytometric markers show specific changes as the COVID-19 disease gets worsened. Chinese diagnostic criteria show fever/respiratory symptoms or decreased/normal white blood cell count and decreased lymphocyte count. Additional criteria include computerized tomography-based pneumonia along with contact with a suspected person suffering from the disease or travel history [Citation8].
Other diagnostic tools like X rays and CT scans and chest ultrasounds also have diagnostic value but are not established as effective in terms of cost, logistics, and expertise availability as well as a prognostic tool in disease management [Citation9]. Nucleic acid detection through samples taken through nasopharyngeal swabs serves as a gold standard. However, limitations include 20% false-negative rates, experienced staff, high cost and turnaround time (3–4 h), and limited access to underdeveloped areas. Moreover, approximately 50% of COVID-19 patients are asymptomatic and pre-symptomatic carriers. Hence, continuous surveillance and contact tracing could help detect such patients and carriers [Citation10].
A complete blood count serves as the most accessible, potent, and readily available test as most routine laboratories are equipped with hematology analyzers. Routine hematological and biochemical parameters have also shown changes in COVID-19 patients, as depicted in various studies. Various hematological parameters in a complete blood count show changes as the disease worsens. COVID-19 infection manifested leukocytosis, leukopenia, lymphocytopenia, eosinopenia, neutrophilia, thrombocytopenia, and raised D-Dimers, Ferritin, CRP, LDH, pro-calcitonin, ALT, AST, PT, APTT, and these parameters are widely used for risk stratification [Citation11–13]. Hence, knowledge about the prognosis of infection and its relevance to comorbidities could provide valuable information on risk stratification and decision making in severely affected COVID-19 patients [Citation14].
This study aimed to evaluate and review variations while analyzing data from COVID-19 and comparing it with healthy controls to determine changes induced in different hematological parameters. Novel inflammatory markers NLR and PLR were also explored to help predict the outcome of COVID-19 infection. Moreover, they were defined as significant markers in different infections and inflammatory conditions in the literature [Citation13]. NLR was first presented in the chemotherapeutic response of esophageal carcinoma in 2012 by dividing the relative percentage of neutrophils by lymphocytes [Citation12]. The normal range should be <3 in healthy population but >3 value, suggests an ongoing infection, and a ratio >9 reveals sepsis. The cut-off value was set at 4 [Citation13]. NLR seems to be associated with inflammation as reported in the literature. NLR was also identified as a prognostic marker of neurological deterioration after acute cerebral hemorrhage, [Citation15], as a marker of glucose control in type 2 diabetics in addition to HbA1c [Citation16], and as an independent prognostic marker of symptomatic hemorrhagic transformation (sHT), a complication of acute ischemic stroke (AIS) [Citation17]. Additionally, serving as a disease predictor tool in inflammatory bowel disease [Citation18] along with serving as a significant predictor of outcomes of various coronary arterial diseases and malignancies. There is overt inflammation in all these diseases, just like COVID-19 infection.
PLR (platelet count divided by lymphocyte count) comes out in the range of 50–150, with mean values varying in the population [Citation7,Citation19] While asymptomatic or moderately affected patients or recovered one had lower values in comparison, coinciding with recently reported studies. Alternatively, a decreased lymphocyte count (lymphocytopenia) was noted in critical phase patients. Consequently, elevating the platelet-to-lymphocyte ratio among critical ICU patients. Interestingly, the ratio declined in the initial stages of disease (at the time of admission to isolation wards); hence supporting the notion of platelet-to-lymphocyte ratio as an independent predictor of mortality and prognosis in critically patients in parallel with the outcomes of recent studies [Citation20,Citation21]. Biochemical parameters including D-dimers, Ferritin, Lactate dehydrogenase, C-reactive protein, Interleukin-6, Alanine transaminase, Aspartate transaminase, and prothrombin time, activated partial thromboplastin time of COVID-19 patients were also compared between a non-severe and severe group of COVID-19 infected patients to find their predictive significance in clinical practice.
In the present study, we aimed to observe the hematological and biochemical parameters of the patients with Covid-19 infection, comparing them with healthy controls. illustrates the graphical abstract of the current study.
2. Materials and methods
2.1. Study design
We conducted a double-centered, hospital-based comparative retrospective case study from April 2020 to April 2021 in the Department of Pathology, Sahiwal Medical College. At the time of analysis, we followed the hospital course along with the outcome of the disease. We followed the ‘Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)’, [Citation22,Citation23] and ‘Standards for Reporting Diagnostic accuracy Studies STARDS’ guidelines for reporting of diagnostic studies to examine the ability of medical tests to correctly categorize the participants of study having that target medical condition [Citation24].
2.2. Patients
Study participants included cases and controls, tested positive and negative for COVID-19 through real-time reverse transcriptase-PCR by nucleic acid detection of sample swabs from the oro-nasopharynx, respectively. These patients were admitted to various isolation wards of tertiary care hospitals in the Sahiwal division, Pakistan, namely District Head Quarter Hospital (DHQ) and GHAQ, Sahiwal. All patients and healthy controls were of Pakistani ethnicity. The control group was observed for three weeks, and they showed no severe or apparent symptoms of respiratory infections. The following variables were recorded for each COVID-19 patient: age, gender, ethnicity and associated comorbidities assessment of disease severity on admission, laboratory findings at the start (the first day of hospital admission), and subsequently during the hospital stay. We followed discharged and recovered patients by telephone and appointments.
2.3. Laboratory procedures and parameters
About 3 ml of venous blood was collected from each COVID-19 positive individual in an EDTA vacutainer tube on the 2nd day of admission of patients to the hospital. Only the reports of the first CBC’s of the cases on the first day of admission to the hospital were considered. A complete blood count (CBC) was performed using the Automatic Hematology Analyzer Swelab Alfa Standard (Boule Medical AB, Sweden). Biochemical parameters were measured for some patients using Beckman Coulter Chemistry Analyser AU680 (Beckman Coulter, Inc, Japan), Semi automated Chemistry Analyser, Microlab 300, Merck, and Wiener Lab Coagulation Analyser Fibrintimer 4. The integrity of reagents and samples was regularly monitored. On admission, patients with COVID-19 were categorized into four groups: (mild, moderate, severe, critical) according to the National Institutes of Health (NIH) classification based on disease severity. Because of the large number of patients, associated comorbidities were retrieved for only severe and critical individuals and those having ICU admission. The hematological parameters, including WBC's, lymphocyte count and percentage, monocyte count and percentage, neutrophils count and percentage, hemoglobin, RBC's and platelets count of both positive and negative patients, were compared.
2.4. Exclusion, inclusion criteria
All the patients diagnosed with COVID-19 through real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) were included in the cases. In contrast, all the healthy individuals screened negatively through rRT-PCR were included in the control group. Individuals <18 years and those having missing data or died before admission to the hospital were excluded from both cases and controls. Additionally, patients with known hematological abnormalities and other inflammatory conditions were also not included.
2.5. Diagnostic criteria
Fever, oxygen saturation, respiratory rate, and lung infiltrates were the clinical parameters to stratify the disease's severity. According to the ‘novel coronavirus infected pneumonia treatment scheme-Sixth edition’ issued by the National Health Commission of the People’s Republic of China [Citation11], the condition of the patients at the time of admission was classified as
‘the regular group (symptoms related to respiratory tract infection, fever, and radiographic evidence of pneumonia) including mild and moderate group patients.
the severe group [meet any of the following criteria]:
at a quiet rest, peripheral blood oxygen saturation is less than 93%;
pulmonary imaging indicated that the lesion progression was more significant than 50% within 24–48 h and at a quiet rest, peripheral blood oxygen saturation is less than 93%;
shortness of breath, a respiratory rate of more than 30 breaths per minute;
PaO2/FiO2 of 300 mmHg or less.
the critically ill group [satisfy any of the following]:
complicated with vital organ failure requires ICU treatment.
shock;
respiratory failure occurs, and mechanical ventilation support is required’ [Citation11];
This study also agrees with the principles of the Declaration of Helsinki of the World Medical Association.
2.6. Statistical analysis
We used the IBM SPSS Ver. 25 and Med Calc Ver. 15.8 statistical package programs for data analysis. We determined the categorical variables of the patients by using the Analysis of Variance which was expressed as a number and percentage n (%). To assess the normality of the data distribution, the Shapiro–Wilk test, Q–Q plots, and histogram were used. Continuous variables were reported as mean and standard deviation, and categorical variables were described as frequency and percentage. Median and interquartile range (IQR) were described by applying the Kruskal–Wallis test. For parametric continued variables, two-tailed independent samples t-test was used for analysis, and they were presented as mean ± standard deviation. Nonparametric variables were analyzed using the Mann–Whitney U-test. Correlations between variables were examined using Spearman's rank correlation analysis. A receiver-operating characteristic (ROC) curve was also generated to determine the efficacy of different parameters in distinguishing COVID-19 patients from healthy controls. The area under the curve (AUC) was calculated. The 95% CI was calculated whenever appropriate, and a two-tailed p < 0.05 was considered statistically significant. Various comparisons among different groups were also performed using the Bonferroni adjustment method. Binominal logistic regression is used to adjust for gender as a confounder. Differences between the two groups were compared using the Mann–Whitney U-test. The significant (p < 0.05) results from the Mann–Whitney U-test (with post-hoc Bonferroni correction) were analyzed.
3. Results
In our study, three hundred seventeen (n = 317) patients diagnosed with COVID-19 after testing positive with PCR for SARS-CoV-2 were enrolled. The comparative control group n = 157 includes healthy individuals who tested negative for COVID-19 through rRT-PCR, and they had no respiratory tract infection and other related symptoms.
3.1. Demographical characteristics
To examine the demographical characteristics of the patients, we executed descriptive statistics for calculating the frequencies and percentages as shown in . Descriptive statistics helps to summarize the data understandably and comprehensively. summarizes the frequencies and percentages of respondents’ demographical characteristics. As visible that, a total of n = 317 (68.0%) positive cases had majority of males n = 198 (62.5%), and n = 111 (35.0%) were females (M = 1.71 ± 0.457) Furthermore, in the control group, we had n = 157 participants. Out of 157 control subjects 111 (70.7%) were female, and 46 (29.3%) were male (M = 1.36 ±0.481). Moreover, regarding the age of our study sample, most of the patients were of the age 26–33 years (n = 102 or 21.5%) (M = 3.46 ±1.75). The age group of controls ranges from 18 to 45 years and it was matched regarding the age and sex. Besides, we also executed the Analysis of Variance to examine any potential mean differences based on the participants’ demographical characteristics. As visible in , based on the gender of both the groups (cases, controls), we did not find any discrepancies in the sampled variables (p ≥ .000). Likewise, we did not find any mean differences based on age of the study participants. Therefore, the Analysis of Variance results showed that there were no demographical differences in the responses.
Table 1. Summary of participants’ demographical data and analysis of variance.
shows the percentages of individuals with comorbidities and their outcome as survived or deceased. Out of 260, who survived, 102 (39.2%) were hypertensive while 22 (36.6%) out of 57 deceased patients were hypertensive. Further, 99 (38%) survived and 13 (21.6%) of the deceased patients had diabetes mellitus.
Table 2. Comorbidities in COVID-19 patients and their association with mortality.
3.2. Hematological parameters of cases and controls
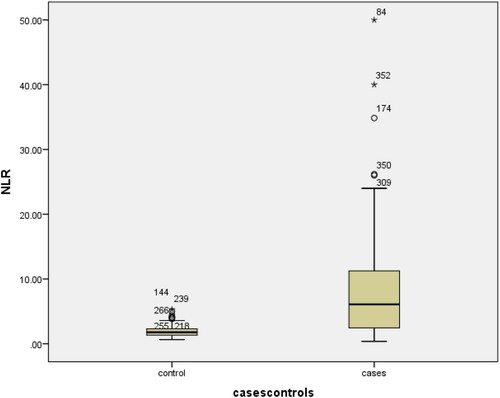
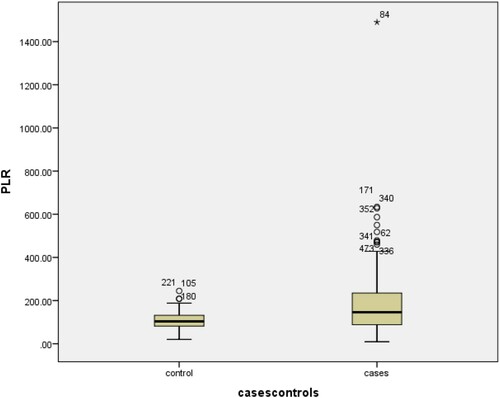
shows a statistically significant difference (p-value <0.001) was found in all the mentioned CBC markers except monocytes, hemoglobin and RBCs. White blood cells measured in 474 patients showed a significant elevation (leukocytosis) (p < 0.001) in the Positive group (m = 11.53, ±7.3) as compared to the control group (7.74 ± 2.21). Lymphocytopenia was seen in cases (M = 1.7, ±1.63) as compared to controls (1.71 ± 1.63) (p < 0.001). Interestingly, Neutrophilia was prominent in COVID-19 affected patients (9.16 ± 6.44) as compared to controls (4.62 ± 1.66) (p < 0.001). Monocyte showed no significant differences in the cases and controls (p = 0.833). Hemoglobin levels were not statistically different among study groups (p = 0.66). Novel hematological parameters, Neutrophil to Lymphocyte ratio (NLR) (reference range: 1–3) and Platelet to Lymphocyte ratio (PLR) (reference range: 36.63–149.13 in males and 43.36–172.68 in females) were also determined for both cases and controls, and the mean of both these ratios was found to be raised in our patients compared to controls (p < 0.001), as depicted in . Similarly, a comparison of NLR and PLR in cases and controls shows significant differences ( and ).
Table 3. Mann–Whitney U-test to examine the laboratory parameters of cases and controls.
3.3. Biochemical parameters determination in cases compared to SI normal values
Due to limited resources, not all the biochemical parameters were measured in healthy controls, and the values of Ferritin, D-Dimer, CRP, IL-6, ALT, AST, PT, APTT, Pro-calcitonin were measured in a limited number of cases and compared with international reference ranges as shown in . The missing values were excluded while analyzing the data. All these parameters showed significant differences. Further, significantly elevated ferritin levels >300 ng/ml (816.24 ± 734.2, p < 0.001) were found more commonly in critical individuals. Coagulation Profile including markedly raised D-dimer levels >550 ng/ml (1624.9 ± 2067.2, p < 0.001), thrombocytopenia (platelet count <100 × 109 cells and prolonged prothrombin time >16 s; p = 0.049) and APTT (36.0 ± 2.92, p < 0.001) were seen in cases as compared to normal values. Further, CRP (49.73 ± 53.59, p < 0.001), IL-6 (24.55 ± 27.99, p < 0.001) and LDH (normal range=120–246) also show a significant increase (845.83 ± 424.8; p < 0.001).
Table 4. Biochemical parameters in COVID-19 positive patients.
3.4. ROC curve analysis between significant markers
Due to statistically significant difference between cases and controls in the CBC markers, Receiver-Operating Characteristics curve analysis was done. As shown in and and . The cut-off point for platelet values was found to be ≥0.0039 (AUC = 0.682; p < 0.001; 95% CI: 0.260–0.368). The cut-off point for hemoglobin was found to be >13.6 (AUC = 0.495; p < 0.001; 95% CI: 0.436–0.554). The cut-off point for WBC’s was found to be ≤7.55 (AUC = 0.647; p < 0.001; 95% CI: 0.705–0.59). The cut-off point for neutrophil values was found to be ≤4.65 (AUC = 0.741; p < 0.001; 95% CI: 0.688–0.790). The cut-off point for NLR was found to be ≤2.98 (AUC = 0.837; p < 0.001; 95% CI: 0.796–0.878). The cut-off point for PLR values was found to be ≤104 (AUC = 0.667; p < 0.001; 95% CI: 0.612–0.721). The cut-off values for significant markers including 1/Platelets, WBC’s, Neutrophils, NLR, PLR and 1/LYMPHOCYTES in the prediction of COVID-19 positive patients were also recommended as shown in the . Additionally, the comparison of results according to cut-off points was also performed ().
Figure 4. Receiver-operating characteristic curve for significant markers in the prediction of COVID-19 positive patients.
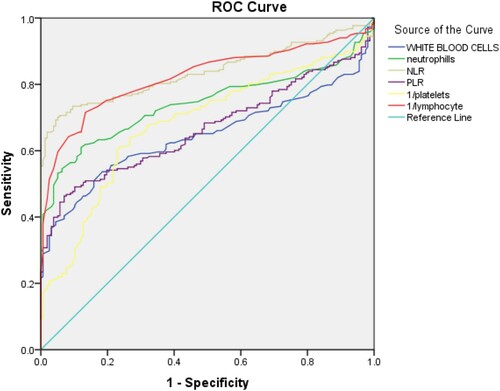
Figure 5. Receiver-operating characteristic curve for significant markers in the prediction of COVID-19 negative patients (controls).
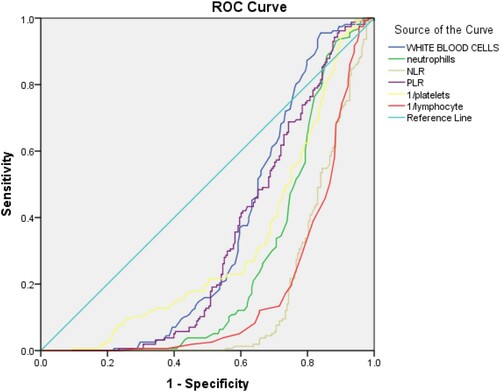
Table 5. Comparison of hematological parameters among patients of COVID-19 disease based on severity.
Table 6. Recommended cut-off values for significant markers in the prediction of Coronavirus Disease of 2019 (+) patients.
Table 7. Comparison of results according to cut-off points.
4. Discussion
The present study showed that hemogram parameters and hemogram-derived indices; such as NLR and PLR of the COVID-19 subjects were significantly different from those in healthy controls.
COVID-19 disease has become a serious health concern for the whole world since it emerged from Wuhan in 2019 [Citation1]. After being declared a global pandemic by WHO, it has affected millions of people, causing huge numbers of deaths and exhaustion of global health resources in our fight against COVID-19. The scarcity and limitation of medical equipment and resources have been felt all around the globe, even in the developed countries, due to immense pressure on the health systems as a result of the rapidly spreading nature of the disease and the resultant patient burden [Citation6,Citation25]. Due to this, it is of immense importance to diagnose the disease timely and effectively with readily available resources so that wastage of time and medical resources and technology can be avoided and the patients of COVID-19 can be triaged quickly for further management. In developing countries like Pakistan, the COVID-19 disease burden on the health facilities was felt even more severely due to the already shortage of equipment and low capacity of the health facilities in diagnosing and managing the patients.
This scarcity of testing facilities and patient management expertise and equipment is even more significant in small towns and cities away from significant metropolitans of the country where the health resources are segregated. Sahiwal, the focus of our study population, is also a small peripheral city of Punjab where many different commonly used COVID-19 standard reference diagnostic tests, developed to get a reliable diagnosis, like Nasopharyngeal swab rRT-PCR (gold standard), CT-scan, and chest x-ray, are not in common reach of the general population. This is due to the limited facilities and diagnostics centers, where these tests are available. Additionally, due to limited human resource to properly conduct and interpret the results and expensive bills which the low income and poor population cannot afford. The equipment availability and its sustainability and maintenance are not usually met regularly because of the need to get supplies of parts, films, and test kits from larger cities, creating a dangerous gap interval in which tests could not be performed. This scenario, which is the prevalent case in many Pakistan and many countries of the world, creates an urgent need for an alternate way to diagnose and triage the COVID-19 patient and monitor the prognosis. Therefore, the development of easy to assess and inexpensive markers for the severity of COVID-19 disease has utmost interest. CBC (complete blood counts) is a commonly used and readily available test in medical laboratories everywhere. They are widely used in clinical practice serving as a routine lab test as they are performed easily and quickly, require little medical expertise to perform and interpret results, and are also easily available and cost-effective providing significant prognostic and diagnostic value in many clinical conditions [Citation26]. NLR and PLR may yield such advantages in the pandemic era, which have been shown to be increased in COVID-19 patients compared to the control subjects in the present study.
Various biomarkers in expanded CBC's have been proved to serve as valuable tools in diagnosing various infectious diseases, including COVID-19. Several studies have associated leukocytosis, lymphopenia, and neutrophilia with the diagnosis of COVID-19 [Citation21]. In our study, leukocytosis was also significant in confirmed patients (cases). These results were consistent with a study conducted in China which showed that leukocytosis was associated with an increased risk of death in the hospital setting [Citation27]. Zhou et al. also noted that leukocytosis was more common in deceased patients of COVID-19 [Citation28]. Further, lymphopenia and neutrophilia were mentioned in the guidelines released by Australia and New Zealand [Citation29].
Regarding age and gender, we did not find any differences among the study participants. Therefore, the results of the Analysis of Variance showed that there were no demographical differences in the responses. Moreover, mortality rate was found in 15.8% in our study, which is much greater than the overall disease mortality reported in Pakistani population. But this could be a false negative because it only projects the mortality among the admitted patients in our hospital.
Additionally, our results showed lymphopenia in cases as compared to the healthy controls (). CDC and Huang et al in China have identified lymphopenia as the most common finding in COVID-19 affected patients [Citation30,Citation31]. Studies show that the leucocyte and lymphocyte count is normal in asymptomatic patients and also when the COVID-19 virus is in its incubation period in a person. SARS-COV-2 infects cells of gastrointestinal tract (GIT), heart, and lungs that express ACE-2 receptors following viremia [Citation32] As lymphocytes also express ACE-2 receptors, the virus causes their lysis. After one to two weeks, there is a surge in cytokines producing ‘cytokine storm’, lymphopenia then becomes more prominent due to atrophy of lymphoid organs and hence their decreased production and turnover [Citation33]. Hence, lymphopenia is identified as one of the most important prognostic markers in COVID-19 patients [Citation34]. Our results are consistent with a study conducted in 148 patients in Singapore in which neutrophilia was reported most commonly in serious COVID-19 patients requiring hospital admission (11.6 × 109 /L vs. 3.5 × 109 /L) [Citation26]. Gong et al. [Citation35] and Qin et al. [Citation36] also identified neutrophilia in critical and severe patients (p < 0.001). Similarly, Li et al. also reported neutrophilia in non-survivors more commonly than in the survivors of COVID-19 infection [Citation7] The possible cause of neutrophilia may be the ‘cytokine storm’ as the mean value of IL-6 is also markedly raised, justifying a significant ‘cytokine storm’. Additionally, the superimposed bacterial infection and drugs used in the treatment can also cause this presentation.
Raised LDH levels in our patients can be explained by the coexisting lactic acidosis, specifically present in cancer patients and patients with comorbidities like heart disease, hence increasing associated complications (). This further inhibits lymphocyte proliferation. Consequently, decreased lymphocyte count should be monitored and vigilantly gauged regularly in COVID-19 patients to determine disease progression [Citation37].
Moreover, NLR and PLR ratios also play an essential role in diagnosing COVID-19 disease [Citation38]. Our results of NLR and PLR show a significant difference between cases and controls. Since, there is a strong systemic inflammatory response in COVID-19 infection, virus-induced inflammatory markers IFN-ϒ, IL-8, IL-6, GCSF, TNF-α activates neutrophils. Conversely, helper T-lymphocytes and other immune cells are considerably declined, causing an overall increase in NLR and hence disease progression. Previous investigations have also identified NLR and PLR as valuable prognostic factors for disease progression [Citation39,Citation40].
NLR has been suggested as a prognostic factor in other conditions, too. For example, Sit et al. noted that thyroid nodules patients have increased NLR in the preoperative period serving as a prognostic marker in case of underlying malignant nodular disease [Citation41]. Additionally, in healthy individuals increased NLR, MLR, PDW can be associated with hepatic steatosis [Citation42]. All these conditions are associated with inflammation just as in COVID-19 infection. Similarly, patients with severe COVID-19 infection reported an increased NLR than those with non-severe or mild COVID-19 infection [Citation43,Citation44].
In COVID-19 infection an increase in D-dimer with a minute decrease in platelet count has been observed. In our study, Elevated platelet count (thrombocytosis) was noted in COVID-19 patients at the time of admission to hospital (initial phase of the disease) and admission to ICU (critical phase) and expired patients. Tang et al. noted that among the more concerning features of COVID-19 infection is a coagulopathy characterized by high D-dimer and fibrinogen concentrations with minor changes in prothrombin time and platelet count. This signifies that the ratio of platelet count and d-dimer values changes in the COVID-19 patients, and the results of mean D-Dimer/Platelet ratio as shown in () in our study are consistent with the above study [Citation45] We statistically analyzed this ratio for our cases and found some variations from normal. In our study, biochemical parameters including ferritin, CRP, Pro-calcitonin, and PT also show a significant increase, as shown by Huang et al., suggesting that increased serum CRP, PCT, D-dimer, and ferritin were associated with a poor outcome in COVID-19 and superimposed co-infection [Citation46]. Additionally, in a retrospective study conducted in Wuhan, China, deceased patients from COVID-19 showed a higher LDH (p < 0.0001), increased serum pro-calcitonin (p < 0.0001), elevated IL-6 (p < 0.0001), and raised serum ferritin (p < 0.0008) than non-severe patients [Citation47] Higher ferritin and CRP may be associated with ARDS. Ferritin levels were elevated in our cases (816.24 ± 734.2) (p < 0.0001), which may be related to elevated hepcidin levels due to inflammatory reaction. Kell et al. [Citation48] noted that ferritin levels greater than 600 ng/dL suggest cellular damage. Moreover, CRP is also considered a marker of inflammatory reaction and is raised in COVID-19 as it is an inflammatory condition.
In our findings, the platelet count was decreased in cases compared to controls, but the values were still within normal limits. However, few patients had mild thrombocytopenia, which was comparable with the findings of a study conducted by Liu et al., who noted that most hospitalized patients had normal platelet counts, only a few had decreased platelets [Citation49].
Interestingly, PT and APTT were abnormally elevated due to disseminated intravascular coagulopathy like state as shown by different studies [Citation50]. Moreover, raised PT was associated with raised ARDS and significantly raised D-dimer entails ARDS, sepsis and death. D-dimers are the degradation product of fibrin, and they are routinely used to diagnose thrombotic state. Hence, any inflammatory condition that changes fibrin levels is associated with elevated d-dimers levels [Citation51]. Tang et al. noted that non-survivors have significantly increased D-dimers (p < 0.05), fibrin degradation products (p < 0.05), and PT (p < 0.05), APTT (p < 0.05). Moreover, fibrinogen was significantly decreased consequently with deranged coagulation profile [Citation45]. Hence, DIC and venous thromboembolism are the most common complications, and the results may complement our understanding of this complication [Citation52]. Unfractionated or low molecular weight heparins (LMWH), instead of direct oral anticoagulants (DOACs), should be given due to possible drug-drug interactions with subsequent antibiotic (such as azithromycin) and antiviral (especially anti-HIV protease inhibitors such as ritonavir) treatment [Citation53].
Increased platelet activation and relative lymphopenia due to apoptosis of lymphocyte cause markedly elevated PLR in COVID-19 positive patients. The results of NLR and PLR in our study are consistent with a study conducted by Yang et al. [Citation34]. A study from Pakistan conducted by Asghar et al. [Citation54] reported a mean PLR at the admission to the ward as 169.81 ± 105.30, which was lower than our findings 181 ± 150, which may indicate that there may be variation in inflammatory activity at the time of presentation. Similar to our study, comorbidities including diabetes, coronary heart disease, hypertension, COPD, and asthma were also reported in different studies, predicting the severity of COVID-19 infection [Citation2,Citation36]. Additionally, associated comorbidities increase the risk of DIC, consequently elevating the d-dimers level [Citation55,Citation56]. The deceased group in our study was most commonly affected by hypertension and diabetes.
By the evaluation of the trends and variations of CBC parameters in the patients of COVID-19 and their comparative analysis with CBC parameters of the controls, we have concluded that by using a commonplace and easily available test like CBC (index test) we can find reliable evidence of the presence of COVID-19 disease in the absence or unavailability of other reference standard tests like rRT-PCR, CT-scan, etc. This can be extremely useful in the response of our health systems against the COVID-19 pandemic in developing countries and areas which are struggling with increasing patient burden due to limited resources and weak health infrastructure, CBC parameters being commonly available in the majority of the medical laboratories, even smaller ones. We used the ROC curve to find the cut-off values of different individual CBC parameters in predicting COVID-19 disease successfully ( and ), so that the information derived from a regular CBC report can be easily analyzed and applied for COVID-19 triage, even by a person with limited medical expertise and background knowledge. We also derived sensitivity and specificity measures of these parameters to strengthen our argument of effective application of this data for practical purposes in clinical scenario ( and ). We compared the values of CBC parameters in confirmed COVID-19 patients, classified according to their severity, and concluded the effectivity of our index test in predicting and giving valuable information about the prognosis and nature of the disease. Individuals were divided into four groups based on their severity levels mild, moderate, severe, and critical, as shown in according to Chinese criteria [Citation11]. shows the comparison between non-severe (including mild, moderate group) and severe (including severe and critical group). WBC's, neutrophils NLR, LDH, PLR CRP showed a statistically significant difference among severe and non-severe groups.
Figure 6. Changes in the parameters based on the severity level of cases compared with normal values in controls.
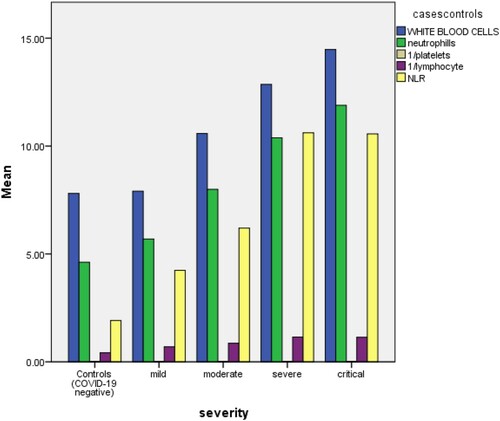
We have also inferred that expanded CBC parameters can provide very significant information about the prognosis and nature of the disease. This makes the index test of this study beneficial in special settings in response to COVID-19. We recommend exploring the potential CBC markers in COVID-19 as current literature is extremely deficient in this respect. To our knowledge, this is the first study from Pakistan, reporting findings on the comparison of hematological and biochemical parameters of COVID-19 patients. Adoption of expanded CBC test (index test) in the above-discussed context can prove to be very helpful in our opinion.
5. Limitations of the study
Small study population is a limitation of the present study. Moreover, the study cohort included only patients presented to the tertiary care hospitals of the Sahiwal division as the overall case rise in Sahiwal was gradual as compared to other districts of Punjab, Pakistan.
6. Conclusion
COVID-19 shows significant hematopoietic changes associated with a hypercoagulable state. Careful monitoring of all the CBC-associated hematological and biochemical parameters can assist clinicians in identifying and determining the course of the disease, which can help provide prompt treatment and ICU admission to those in need. It will elevate the burden on the health system its resources, especially in countries with limited resources. The patient outcome can be further improved by continuous vigilance and preventing hypercoagulation and DIC by thromboprophylaxis. The abnormal hematopoietic values not only serve as a diagnostic and prognostic value in determining the course of the disease but also the outcome and severity of COVID-19 infection. Overall mortality and morbidity can be lowered in critical patients and those having comorbidities. We suggest that, due to their inexpensive and easy to assess nature, elevated NLR and PLR could be useful in the diagnosis of COVID-19 along with other reference tests, especially when clinical suspicion continues despite negative PCR results.
Acknowledgements
The authors are indebted to all the healthcare professionals, nurses, and patients who are fighting with COVID-19 together. A special thanks to Dr. Waseem, Assistant Professor Pulmonology, DHQ Teaching Hospital Sahiwal, Dr. Tania Shakoori, Associate Professor, Institute of Molecular Biology and Biotechnology, University of Lahore, Dr. Rana Amir Diwan, Head of Community Medicine, Sahiwal Medical College, Sahiwal & Dr. Akram Riaz, Department of Psychology, University of Lahore. Author contributions: Atiqa Khalid and Muhammad Ali Jaffar conceptualized and designed the study, collected data, and constructed a first draft. Atiqa Khalid also planned methodology, administered project & took responsibility for the article. Sana Ali, Tabinda Khan and Gulali Aktas conducted literature search, elaborated arguments and contributed to subsequent drafts of the paper. Raees Abbas Lail, Abdul Waris, Aamnah Javed and Nouman Ijaz helped in data collection. Muhammad Nasir contributed towards literature search. All authors revised the document for critical intellectual input, and all authors approved the final version. Compliance with ethical standards: Data Set: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Consent for publication: Informed consent was granted from all the study participants and sources whose data and figures were used. Informed consent: Informed consent was received from all the participants whose data are utilized. Ethical Approval: This study was carried out in line with research regulations, including approval by the Sahiwal medical college ethical committee (vide letter SLMC/DME/103) and Government of Pakistan. This study is in accordance with the principles of the ‘World Medical Association Helsinki Declaration’.
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Khalid A, Ali S. COVID-19 and its challenges for the healthcare system in Pakistan. Asian Bioeth Rev. 2020 Dec;12(4):551–564.
- Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941.
- Xie X, Zhong Z, Zhao W, et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343.
- Li D, Wang D, Dong J, et al. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep-learning-based CT diagnosis and insights from two cases. Korean J Radiol. 2020;21(4):505.
- Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 Aug;296(2):E32–E40.
- Di Gennaro F, Pizzol D, Marotta C, et al. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Public Health. 2020 Apr 14;17(8):2690. DOI:https://doi.org/10.3390/ijerph17082690.
- Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020 Jul;99(7):1421–1428.
- Protocol on prevention and control of COVID-19 (Edition 6) [Internet]. [cited 2021 Mar 20]. Available from: http://en.nhc.gov.cn/2020-03/29/c_78468.htm.
- Wang R, Hozumi Y, Yin C, et al. Mutations on COVID-19 diagnostic targets. Genomics. 2020;112(6):5204–5213.
- Aktas G. A comprehensive review on rational and effective treatment strategies against an invisible enemy; SARS Cov-2 infection. Exp Biomed Res. 2020 Oct 1;3(4):293–311.
- National Health Commission of the PRC [Internet]. [cited 2021 Mar 20]. Available from: http://en.nhc.gov.cn/.
- Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012 Mar;36(3):617–622.
- Martins EC, da Silveira LF, Viegas K, et al. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev Bras Ter Intensiva. 2019;31(1):63–70.
- Certain medical conditions and risk for severe COVID-19 illness CDC [Internet]. [cited 2021 Jun 29]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017 Aug 22;8(34):57489–57494.
- Duman TT, Aktas G, Atak BM, et al. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019 Apr 18;19(1):1602.
- Świtońska M, Piekuś-Słomka N, Słomka A, et al. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in Ischemic stroke patients undergoing revascularization. Brain Sci. 2020;10(11):771. DOI:https://doi.org/10.3390/brainsci10110771.
- Aktas G, Duman T, Atak B, et al. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Fam Med Prim Care Rev. 2020;22(2):107–110.
- Simadibrata DM, Pandhita BAW, Ananta ME, et al. Platelet-to-lymphocyte ratio, a novel biomarker to predict the severity of COVID-19 patients: a systematic review and meta-analysis. J Intensive Care Soc. 2020: 1–7. DOI:https://doi.org/10.1177/1751143720969587.
- Qu R, Ling Y, Zhang Y-H-Z, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020 Sep;92(9):1533–1541.
- Waris A, Din M, Khalid A, et al. Evaluation of hematological parameters as an indicator of disease severity in covid-19 patients: Pakistan’s experience. J Clin Lab Anal. 2021;35(6):e23809. DOI:https://doi.org/10.1002/jcla.23809.
- Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499.
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31.
- Korevaar DA, Cohen JF, Reitsma JB, et al. Updating standards for reporting diagnostic accuracy: the development of STARD 2015. Res Integr Peer Rev. 2016 Dec;1(1):7.
- Miller IF, Becker AD, Grenfell BT, et al. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020 Aug;26(8):1212–1217.
- Barger AM. The complete blood cell count: a powerful diagnostic tool. Vet Clin Small Anim Pract. 2003;33(6):1207–1222.
- Xu P, Tian R, Luo S, et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10(14):6372–6383.
- Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–273.
- Weinkove R, McQuilten ZK, Adler J, et al. Managing haematology and oncology patients during the COVID-19 pandemic: interim consensus guidance. Med J Aust. 2020 Jun;212(10):481–489.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506.
- CDC. Healthcare workers [Internet]. Centers for Disease Control and Prevention. 2020 [cited 2021 Jun 29]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):1–5.
- Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690.
- Yang A-P, Liu J-P, Tao W-Q, et al. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504.
- Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833–840. DOI:https://doi.org/10.1093/cid/ciaa443..
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Jul 28;71(15):762–768.
- Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934–943.
- Sit M, Aktas G, Erkol H, et al. Neutrophil to lymphocyte ratio is useful in differentiation of malign and benign thyroid nodules. P R Health Sci J. 2019;38(1):60–63.
- Aktas G, Duman TT, Kurtkulagi O, et al. Liver steatosis is associated both with platelet distribution width, neutrophil/lymphocyte and monocyte/lymphocyte ratios. Prim Health Care Open Access. 2020;10(4):1–4.
- Chan AS, Rout A. Use of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19. J Clin Med Res. 2020 Jul;12(7):448–453.
- Zeng F, Li L, Zeng J, et al. Can we predict the severity of coronavirus disease 2019 with a routine blood test? Pol Arch Intern Med. 2020;130(5):400–406. DOI:https://doi.org/10.20452/pamw.15331.
- Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020 Aug 3;25(1):30.
- Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734.
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost JTH. 2020 Apr;18(4):844–847.
- Huang I, Pranata R, Lim MA, et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175.
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020 Jul;95(7):834–847.
- Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748–773.
- Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020 May 18;31(4):490–496.
- Wang L, He W-B, Yu X-M, et al. Prolonged prothrombin time at admission predicts poor clinical outcome in COVID-19 patients. World J Clin Cases. 2020;8(19):4370.
- Linkins L, Takach Lapner S. Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol. 2017;39:98–103.
- Liao D, Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020 Sep 1;7(9):e671–e678.
- Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026.
- Asghar MS, Khan NA, Haider Kazmi SJ, et al. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: a retrospective comparative analysis. J Community Hosp Intern Med Perspect. 2020 Oct;10(6):514–520.
- Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(1):122–124.
- Yao Y, Cao J, Wang Q, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020 Dec;8(1):49.