ABSTRACT
Background
Azacitidine (AZA) is the standard of care for higher-risk myelodysplastic syndrome (HR-MDS) patients ineligible for intensive therapy. Clinical outcome discrepancies reported in clinical trials and real-life settings stimulate the search for new prognostic factors.
Methods
We retrospectively evaluated 315 MDS, 20–30% blast acute myeloid leukemia (AML) and chronic myelomonocytic leukemia (CMML) patients treated with azacitidine in 12 centers cooperating within the Polish Adult Leukemia Group (PALG).
Results
The median number of AZA cycles was 7 (1–69) and 24% patients received fewer than 4 cycles (early failure, EF). Serum albumin level was an independent predictor of EF occurrence. Complete remission (CR) was obtained in 20% and partial remission (PR) in 12% of patients. Hematologic improvement – erythroid (HI-E), neutrophil (HI-N), or platelet (HI-P) was achieved in 51%, 36%, and 48% of patients, respectively. No factors significantly predicted CR or PR in the multivariate analysis. For HI-E and HI-P, lower LDH level predicted response. Median survival was 15 (13–19) months. Lower serum albumin level, serious infection and receiving <4 AZA cycles independently predicted a worse overall survival (OS) (p < 0.05).
Conclusion
Serum albumin assessment before azacitidine treatment can help to identify patients with higher risk of early failure and worse clinical outcome.
1. Introduction
Azacitidine is the standard of care for patients with higher risk myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) and chronic myelomonocytic leukemia (CMML) who are not eligible for intensive chemotherapy or hematopoietic stem cell transplantation. A large randomized trial AZA-001 showed survival benefit of azacitidine treatment when compared with conventional treatments with a median survival 24.5 months [Citation1]. On the other hand, overall survival was shorter in smaller randomized trials and real-life studies (15–20 and 13–17 months, respectively) [Citation2–4]. In this context, both pre-treatment as well as on-treatment risk stratification and identification of factors predicting response and prognosis seem to be of special value. It can be especially helpful for ‘borderline’ candidates for allogeneic transplantation at the age of 60–75 years with comorbidities. Many previous studies analyzing prognostication of azacitidine-treated patients focused only on selected parameters. The present study takes advantage of a large multicenter cohort of MDS/AML/CMML azacitidine-treated patients and analyzes in a complex way the patient-, disease-. and treatment-related factors that might affect the clinical outcome.
2. Patients and methods
2.1. Patients
The study included 315 consecutive patients with higher-risk MDS (defined as intermediate-2 or higher IPSS score), acute myeloid leukemia with 20–30% bone marrow blasts, and chronic myelomonocytic leukemia 2 (CMML 2) based on WHO 2008 classification. In addition, patients were ineligible for stem cell transplantation at that time due to the stage of disease, performance status, age or comorbidities. Data of patients treated between December 2008 and February 2019 were retrospectively collected by 12 Polish hematologic centers cooperating within the Polish Adult Leukemia Group. Baseline patient demographics, ECOG (Eastern Cooperative Oncology Group) performance status, red blood cell transfusion dependency (RBC TD) status, disease characteristics including morphologic assessment according to WHO 2008 classification of bone marrow cells, peripheral blood counts and selected serum biochemical data were recorded [Citation5]. Bone marrow cellularity was assessed in biopsy samples and hypocellularity was defined as <30% cellularity [Citation6]. Cytogenetic abnormalities were documented according to the International System of Human Cytogenetic Nomenclature recommendations and classified using IPSS and IPSS-R scores [Citation7–9]. In 20–30% marrow blast patients IPSS and IPSS R scores were also calculated. Monosomal karyotype was defined as the presence of at least two autosomal monosomies or one autosomal monosomy associated with at least one structural abnormality [Citation10]. Comorbidities were assessed based on hematopoietic cell transplantation-specific comorbidity index (HCT-CI) and MDS comorbidity index (MDS-CI) [Citation11, Citation12]. Multidrug-resistant bacteria gut colonization was defined as the presence of bacteria non-susceptible to at least one agent in three or more antimicrobial categories in the gut flora [Citation13].
2.2. Treatment and response categories
Azacitidine was administered subcutaneously at a single dose of 75 mg/m2/day for 7 consecutive days, or for 5 days/weekend off/2 days, in 28-day cycles. All patients were routinely evaluated in terms of response after 3–6 cycles based on complete blood count and marrow aspirate according to International Working Group (IWG) 2006 criteria [Citation14]. Overt progression as well as any hematologic improvement in patients who received less than 4 cycles were also recorded.
2.3. Statistical analysis
In the statistical analysis, standard survival techniques were used, i.e. Kaplan–Meier estimator with a log-rank test and Cox regression model. Possible causal relationships were expressed using the hazard ratios with 95% confidence intervals and p values. The effects of selected categorical variables were displayed graphically as survival curves. Regression analysis of the determined risk factors was carried out in univariate and multivariate approaches. The computation was performed using R statistical platform [R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www. R-project.org/].
2.4. Compliance with ethical standards
The study was performed in accordance with local ethics committee guidelines and did not require approval by the ethics committee because of its retrospective nature.
3. Results
3.1. Patient characteristics
We collected data on 315 patients treated with azacitidine: AML with 20–30% bone marrow blasts, n = 66 (21%); MDS, n = 210 (67%); CMML, n = 39 (12%) (). Median age was 69 years (54 years in patients referred for allo-SCT), 61% were male. Patients with available IPSS were in the intermediate-2 (58%) and high (42%) risk categories. In 12% of patients, IPSS was not precisely determined due to lack of one out of three mandatory components. Median duration of MDS/AML/CMML before the start of azacitidine treatment was 5 weeks, 18% of patients had previously received chemotherapy – low-dose cytarabine. Among 276 (88%) patients with available cytogenetic results, 113 (41%) had a normal karyotype. The IPSS-R cytogenetic risk included 7 (2%) patients with very good risk, 130 (47%) patients with good risk, 55 (20%) patients with intermediate risk, 64 (23%) with poor risk and 20 (7%) patients with very poor risk. The most frequent cytogenetic abnormalities were: complex karyotype in 57 (21%), monosomal and complex karyotype in 44 (16%), isolated trisomy 8 in 15 (5%), isolated del 5 in 6 (2%), isolated del 7 in 7 (2%), isolated monosomy 7 in 8 (3%) and deletion 20 in 7 (2%).
Table 1. Baseline patient characteristics.
3.2. Treatment modalities, complications and response
The median number of treatment cycles was 7 (1–69) (). Seventy-six (24%) patients permanently discontinued azacitidine treatment before the fourth cycle and were categorized as early failures (patients referred to allo-SCT were excluded from this subset of cases). The reason for premature treatment discontinuation was early death in 33, severe infection in 17, rapid disease progression in 11, prolonged cytopenia in 9, consent withdrawal in 3, stroke in 1, gastrointestinal bleeding in 1, and ileus in 1. The only factor found to significantly predict early failure in multivariate analysis was serum albumin level (OR, 0.3; 95% CI, 0.13–0.7, p = 0.005). An increase in albumin level by 1 g/dL decreased the risk of EF occurrence by 70%. Schedule modifications were applied in 90 (29%) patients – dose delay in 58 (19%), dose reduction in 17 (5%) and both changes in 17 (5%) patients. Total number of azacitidine cycles used was 3407 and most of them 2827 (83%) were administered in inpatient settings. Antimicrobial prophylaxis was used in 53% of patients. Antibiotics, antifungals and antiviral prophylaxis were received by 37%, 35%, and 21% of the patients, respectively. Grade 3–4 infections occurred in 43% (138 of 315) patients. Red blood cell transfusion dependency, HCT-CI score, and IPSS-R cytogenetics were significant predictors for infection selected for multivariate analysis. A total of 125 patients (39%) experienced grade 3–4 hematologic toxicity. Non-hematologic and non-infectious complications were usually mild but in 8% of patients grade 3 or 4 events were reported, of which the most common were: cardiac arrhythmias (n = 4), bleeding (n = 4), renal failure (n = 4) and hepatic failure (n = 3).
Table 2. Azacitidine treatment – outcomes and infections.
In 27 (8%) patients, allogeneic stem cell transplantation was performed after a reduction in marrow blasts, general state improvement or change of the patient’s decision. In the 280 patients evaluable for response to azacitidine, 20% achieved complete remission (CR), 12% partial remission (PR), 49% stable disease (SD), and 19% experienced progression of disease (PD). Hematologic improvement, including cases with CR and PR classified as erythroid response (HI-E), was observed in 51%, neutrophil response (HI-N) in 36%, and platelet response (HI-P) in 48% of patients. Median time to response was 3 months (1–17). Platelet doubling after the first cycle was documented in 18% of patients.
3.3. Predictive factors for response
Among the patient-, disease-, and treatment-related factors analyzed regarding CR, PR, and HI responses in univariate analysis, the following parameters were found to have a significant predictive value: hemoglobin level, peripheral blood blast presence, bone marrow blast percentage, bone marrow fibrosis, IPSS cytogenetics, IPSS and IPSS-R score, LDH level, MRB gut colonization, RBC transfusion dependence and ECOG performance status (). None of the above factors achieved statistical significance in multivariate analysis. We did separate analysis in terms of hematologic improvements. In univariate analysis obtaining HI-E was significantly associated with IPSS and IPSS-R cytogenetics, IPSS and IPSS-R score and LDH level; however, in multivariate analysis, only increased LDH level remained significant with a low response rate. Achieving HI-N was associated with lymphocyte and neutrophil counts, bone marrow fibrosis and cellularity, IPSS cytogenetics, LDH and ferritin level in univariate analysis, whereas in multivariate analysis only a higher neutrophil count was significantly related with a lower HI-N rate. In univariate analysis, platelet count, IPSS and IPSS-R cytogenetics, IPSS and IPSS-R score, LDH and ferritin level had a predictive value for HI-P. Elevated LDH and platelet count were predictive for lower HI-P response in multivariate analysis.
Table 3. Univariate and multivariate analysis for response.
3.4.1. Survival analysis – results of univariate analysis
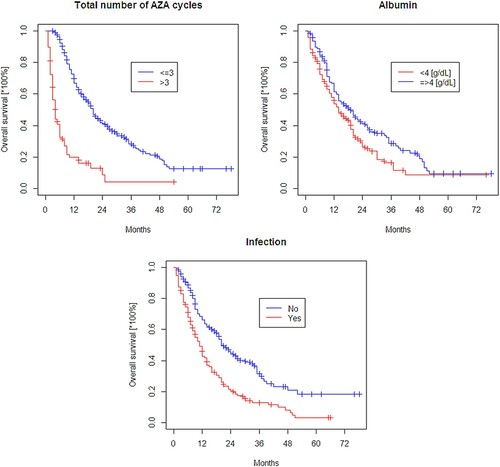
After a median follow-up of 15 months (range, 13–19), 210 (69%) patients deceased and median overall survival was 15 (95% CI, 13–19) months (Supplemental Figure 1). Median survival benefit was observed for patients who achieved response: CR (37 months; 95% CI, 27–NA) or PR (33 months; 95% CI, 20–NA) when compared to patients with SD (16 months; 95% CI, 13–20) or PD (6 months; 95% CI, 6–8); p < 0.001 (Supplemental Figure 2). Survival of patients with hematologic improvement was as follows: HI-E, 30 months (95% CI, 23–40 months); non-HI-E, 7 months (95% CI, 6–10) (p < 0.001); HI-N, 24 months (95% CI, 20–42 months); non-HI-N, 10 months (95% CI, 8–14) (p > 0.05); HI-P, 33 months (95% CI, 22–42 months); and non-HI-P, 8 months (95% CI, 6–10 months) (p < 0.001). Longer survival was observed in patients with albumin level ≥4 g/dL vs. <4 g/dL (19 months vs. 14 months), without infection (20 months vs. 11 months in those with infection), and in patients who received at least 4 azacitidine cycles (20 months vs. 4 months in those treated for up to 3 cycles) (p < 0.001; ). In patients who underwent allo-HSCT following azacitidine treatment, a prolonged survival was observed (27 months vs. 12 months in those without allo-HSCT) (p = 0.003). There was no significant difference in survival in patients treated for 1–3 azacitidine cycles versus at least 4 cycles before allo-HSCT (24 vs. 27 months). The survival of patients with elevated pre-treatment serum LDH level was comparable with those with normal LDH; however, median survival of patients with low LDH (≤0.6 × ULN) was significantly higher than in patients with LDH >0.6 × ULN (47 months vs. 14 months, p = 0.024). The survival of patients with normal karyotype was 23 months (95% CI, 19–30 months). The median survival for the most frequent abnormality findings were: 9 months for complex karyotype (95% CI, 7–14 months), 8–18 months for isolated trisomy (95% CI, 12-NA). The groups with cytogenetics abnormalities were too small to conclude on any statistical significance.
3.4.2. Factors affecting survival – results of multivariate analysis
Based on univariate analysis significance (p < 0.05), the following variables were included in multivariate analysis: age, lymphocyte count, neutrophil count, hemoglobin level, IPSS and IPSS-R cytogenetics, IPSS, IPSS-R score, LDH level, ferritin level, creatinine concentration, MDR gut colonization, RBC transfusion dependency, ECG performance status, number of azacitidine cycles, allogeneic hematopoietic stem cell transplantation, overall response, achieving HI-E, HI-N, HI-P, time to first response, grade 3 or 4 infection (). To make the analyzed group more homogenous, patients who underwent allo-HSCT following azacitidine therapy were excluded from multivariate analysis. In multivariate analysis, less than 4 azacitidine cycles administered (HR, 0.90; 95% CI, 0.88–0.92; p < 0.0005), grade 3/4 infection (HR, 1.82; 95% CI, 1.35–2.45–3.31; p = 0.001) and lower serum albumin level as a continuous variable (HR, 0.67; 95% CI, 0.52–0.87; p = 0.001) retained independent adverse prognostic values.
Table 4. Prognostic factors for overall survival.
4. Discussion
Our work is, to our knowledge, one of the largest studies analyzing in detail the potential factors affecting survival and response in MDS/CML/20–30% blasts AML patients treated with azacitidine. Azacitidine is a disease-modifying agent that prolongs survival of higher-risk MDS patients to 24 months as reported in clinical trial AZA-001, or to 11.6–17 months observed in real-life studies [Citation1, Citation4, Citation15]. The median survival of 15 months in our cohort was similar to other real-life settings but shorter than in the AZA-001 trial. When comparing to the Fenaux study, the frequency of higher risk features was comparable – poor IPSS cytogenetics 34% vs. 28%, the median age was the same, and ECOG ≥2 was 11.4% vs. 7%. On the other hand, we included 18% patients previously treated with cytarabine while in the AZA-001 trial such patients were excluded.
The CR + PR response rate of 32% in our patients was similar to those reported in other studies (23–36%) [Citation2, Citation4]. Up to date, predictive factors for response have not been widely studied. Previous cytarabine therapy, higher BM blasts, lower hemoglobin level and abnormal or poor cytogenetics were reported as poor response predictors [Citation4, Citation16, Citation17]. In our cohort, none of the factors predicting response in univariate analysis remained significant in the multivariate analysis.
Since hematologic improvement plays an important role in MDS treatment outcome, we separated the analysis of predictors for improvement into three lines: erythroid, granulocytic and platelet. Normal/increased marrow cellularity (OR, 4.31; 95% CI, 1.05–17.6; p = 0.042) and presence of fibrosis (OR, 0.54; 95% CI, 0.33–0.88; p = 0.013) correlated with achieving HI-N in univariate analysis. No significant predictive factors for HI-N were found in multivariate analysis, except for neutrophil count. For both HI-E and HI-P, a lower LDH level regarded as a continuous variable was found to be a significant predictive factor for improvement in multivariate analysis. At the same time, LDH level was not an independent prognostic factor for overall survival in multivariate analysis; however, patients with low LDH level (<0.6 × ULN) had longer survival when compared to those with LDH level of ≥0.6 × ULN (47 vs. 14 months, respectively). The group of patients with very low LDH level was rather small as it included 19 patients.
In general, serum LDH is usually elevated in Hodgkin’s and non-Hodgkin’s lymphoma, and it is a strong predictor of survival in the International Prognostic Index (IPI) (Ferraris 1979) [Citation18]. In studies published to date, elevated serum lactate dehydrogenase was found to be a negative prognostic parameter in MDS and AML patients treated with azacitidine and was linked with a higher probability of AML evolution in MDS patients receiving supportive therapy or azacitidine [Citation19–24]. Wimazal et al. reported that in the follow-up of MDS patients, an increase in LDH precedes AML progression [Citation19]. In a recently published study, lower serum LDH levels were associated with HMA responses in CMML patients [Citation25]. However, the exact explanation of the role of LDH as a prognostic as well as predictive factor for HI-E and HI-P in our study remains unclear. Lactate dehydrogenase is a factor of increased turnover and damage of myeloid cells and ineffective hematopoiesis; moreover, lactate dehydrogenase A (a subtype of LDH) has aberrantly high expression in multiple cancers and enhances tumor proliferation, survival, invasion and immune escape [Citation26]. The correlation between serum LDH level and LDH A expression in cancer cells needs further investigation.
Our findings, in contrast to the most studies presented so far, demonstrated that only patient- and treatment-related features have independent impact on survival. Cytogenetics, IPSS-R, IPSS, red blood cell transfusion dependency, marrow and peripheral blood blasts, documented in other series, in the current study were predictive factors for survival but only in the univariate analysis [Citation15, Citation16, Citation27]. We identified baseline serum albumin level as a prognostic variable in both uni- and multivariate analyses. Albumin remained significant as a continuous variable (p = 0.001) as well as a categorical variable. Patients with albumin level ≥4 g/dL had longer median survival than patients with albumin level <4 g/dL (median survival 19 months vs. 14 months, p < 0.05). To our best knowledge, our study is the first one reporting albumin level as a prognostic marker in MDS patients treated with azacitidine. Serum albumin is a well-known surrogate of the general condition, comorbidities (including liver and kidney function), ECOG performance status and nutritional status. It is also an indicator of chronic inflammation. Recently, serum albumin level was found to be an important prognostic biomarker in solid organ malignancies in combination with CRP value and also in allogeneic hematopoietic cell transplantation recipients implemented in indexes: Hematopoietic Cell Transplantation – Glasgow Prognostic Score (HCT-GPS) and Augmented Hematopoietic Cell Transplantation – Comorbidity Index in combination with ferritin level, platelets and comorbidities [Citation28, Citation29]. The Polish Adult Leukemia Group has documented that lower albumin level significantly increases the risk of serious infections in MDS/AML/CMML patients treated with azacitidine [Citation30]. In our series, albumin level remained independent from ECOG, MDS-CI, HCT-CI and ferritin level.
Two other independent prognostic markers were: occurrence of serious infection and number of received azacitidine cycles. These results were in accordance with recently published studies and reinforce the view that well-tailored management plays a very important role in obtaining the best outcome [Citation15, Citation27, Citation30]. In the current study, median survival of patients who prematurely discontinued azacitidine treatment was 4 months as compared to 16 months in those who received at least four cycles. In a large Canadian study, the difference in these subgroups was also significant (10 vs. 18 months, respectively). Reaching the number of at least four azacitidine cycles is substantial due to many reasons, including such as: median time to achieve response is commonly 3–4 months, major complications occur within the first three cycles, lack of treatment worsens the risk of AML progression, and no second-line treatment was proved to be effective so far. In our study, the main reason for premature treatment discontinuation was severe infection. Infectious complications are one of the most significant issues especially during first three AZA cycles as reported by other investigators [Citation31].
In that context, avoiding early failure by adopting a special approach in infection-susceptible patients during the first three azacitidine cycles seems to be of special value.
A weak point of our study is its retrospective nature and a relatively heterogeneous population including MDS, CMML, and AML <30% blasts. Another limitation is the lack of somatic mutational analysis, although the studies published so far have not shown any advantage of molecular analyses over clinical, standard cytogenetics and biochemical data in azacitidine-treated patient prognostication.
The main strength of this study is the analysis of a wide spectrum of clinical and laboratory data in the large, unselected, ‘real-world’ population. In conclusion, this report highlights the importance of patient-related factors in clinical outcome and helps with identifying patients prone to premature treatment discontinuation.
Author contributions
KM, KL conceived the concept, formulated study design, collected, and analyzed data and wrote the manuscript. AT, PS, KKS, ES, AG, WM, AM, AK, MC, SZL, PR, JDS, EW, JH, BP, PC, AWG, RM,GB, TC, JDT collected data, revised, and approved the final version of the manuscript.
Supplemental Material
Download MS Word (234 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study correspondence. Lancet Oncol. 2009;10(3):223–232. doi:https://doi.org/10.1016/S1470-2045(09)70003-8.
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–2440. doi:https://doi.org/10.1200/JCO.2002.04.117.
- Papageorgiou SG, Kontos CK, Kotsianidis I, et al. The outcome of patients with high-risk MDS achieving stable disease after treatment with 5-azacytidine: A retrospective analysis of the hellenic (Greek) MDS study group. Hematol Oncol. 2018;36(4):693–700. doi:https://doi.org/10.1002/hon.2551.
- Voso MT, Niscola P, Piciocchi A, et al. Standard dose and prolonged administration of azacitidine are associated with improved efficacy in a real-world group of patients with myelodysplastic syndrome or low blast count acute myeloid leukemia. Eur J Haematol. 2016;96(4):344–351. doi:https://doi.org/10.1111/ejh.12595.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi:https://doi.org/10.1182/blood-2009-03-209262.
- Mangi MH, Mufti GJ. Primary myelodysplastic syndromes: diagnostic and prognostic significance of immunohistochemical assessment of bone marrow biopsies. Blood. 1992;79(1):198–205. doi:https://doi.org/10.1182/blood.v79.1.198.bloodjournal791198.
- Shaffer LG, Slovak ML, Campbell LJ. ISCN 2009 An International System for Human Cytogenetic Nomenclature (2009). 1 edition Basel: S. Karger; 2009.
- Greenberg BP, Cox C, Lebeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088.
- Greenberg PL, Tuechler H. Revised international prognostic scoring system for MDS. Blood. 2012;120(12):2454–2465. doi:https://doi.org/10.1182/blood-2012-03-420489.The.
- Breems DA, Van Putten WLJ, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: A better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–4797. doi:https://doi.org/10.1200/JCO.2008.16.0259.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi:https://doi.org/10.1182/blood-2005-05-2004.
- della Porta MG, Malcovati L, Strupp C, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96(3):441–449. doi:https://doi.org/10.3324/haematol.2010.033506.
- Magiorakos A, Srinivasan A, Carey RB, et al. Bacteria : an International expert proposal for interim standard definitions for acquired resistance. 2011.
- Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–425. doi:https://doi.org/10.1182/blood-2005-10-4149.
- Mozessohn L, Cheung MC, Fallahpour S, et al. Azacitidine in the ‘real-world’: an evaluation of 1101 higher-risk myelodysplastic syndrome/low blast count acute myeloid leukaemia patients in Ontario, Canada. Br J Haematol. 2018;181(6):803–815. doi:https://doi.org/10.1111/bjh.15273.
- Itzykson R, Thépot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403–411. doi:https://doi.org/10.1182/blood-2010-06-289280.
- Sébert M, Komrokji RS, Sekeres MA, et al. Impact of baseline cytogenetic findings and cytogenetic response on outcome of high-risk myelodysplastic syndromes and low blast count AML treated with azacitidine. Leuk Res. 2017;63(July):72–77. doi:https://doi.org/10.1016/j.leukres.2017.10.013.
- Ferraris A, Giuntini P, Gaetani G. Serum lactic dehydrogenase as a prognostic tool for non-Hodgkin lymphomas. Blood. 1979;54(4):928–932.
- Wimazal F, Sperr WR, Kundi M, et al. Prognostic significance of serial determinations of lactate dehydrogenase (LDH) in the follow-up of patients with myelodysplastic syndromes. Ann Oncol. 2008;19(5):970–976. doi:https://doi.org/10.1093/annonc/mdm595.
- Pleyer L, Burgstaller S, Girschikofsky M, et al. Azacitidine in 302 patients with WHO-defined acute myeloid leukemia: results from the Austrian azacitidine registry of the AGMT-study group. Ann Hematol. 2014;93(11):1825–1838. doi:https://doi.org/10.1007/s00277-014-2126-9.
- Moon JH, Kim SN, Kang BW, et al. Predictive value of pretreatment risk group and baseline LDH levels in MDS patients receiving azacitidine treatment. Ann Hematol. 2010;89(7):681–689. doi:https://doi.org/10.1007/s00277-010-0921-5.
- Germing U, Hildebrandt B, Pfeilstöcker M, et al. Refinement of the international prognostic scoring system (IPSS) by including LDH as an additional prognostic variable to improve risk assessment in patients with primary myelodysplastic syndromes (MDS). Leukemia. 2005;19(12):2223–2231. doi:https://doi.org/10.1038/sj.leu.2403963.
- van der Helm LH, Alhan C, Wijermans PW, et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named p. Br J Haematol. 2011;155(5):599–606. doi:https://doi.org/10.1111/j.1365-2141.2011.08893.x.
- Bernal T, Martínez-Camblor P, Sánchez-García J, et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: results from the Spanish registry. Leukemia. 2015;29(9):1875–1881. doi:https://doi.org/10.1038/leu.2015.115.
- Coston T, Pophali P, Vallapureddy R, et al. Suboptimal response rates to hypomethylating agent therapy in chronic myelomonocytic leukemia; a single institutional study of 121 patients. Am J Hematol. 2019;94(7):767–779. doi:https://doi.org/10.1002/ajh.25488.
- Feng Y, Xiong Y, Qiao T, et al. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018;7(12):6124–6136. doi:https://doi.org/10.1002/cam4.1820.
- Scalzulli E, Molica M, Alunni Fegatelli D, et al. Identification of predictive factors for overall survival at baseline and during azacitidine treatment in high-risk myelodysplastic syndrome patients treated in the clinical practice. Ann Hematol. 2019;98(8):1919–1925. doi:https://doi.org/10.1007/s00277-019-03724-9.
- Shibasaki Y, Suwabe T, Katagiri T, et al. Refinement of the Glasgow Prognostic Score as a pre-transplant risk assessment for allogeneic hematopoietic cell transplantation. Int J Hematol. 2018;108(3):282–289. doi:https://doi.org/10.1007/s12185-018-2463-x.
- Vaughn JE, Storer BE, Armand P, et al. Design and validation of an augmented hematopoietic cell transplantation-comorbidity Index comprising pretransplant ferritin, albumin, and platelet count for prediction of outcomes after allogeneic transplantation. Biol Blood Marrow Transplant. 2015;21(8):1418–1424. doi:https://doi.org/10.1016/j.bbmt.2015.04.002.
- Mądry K, Lis K, Biecek P, et al. Predictive Model for Infection Risk in myelodysplastic syndromes, acute myeloid leukemia, and chronic myelomonocytic leukemia patients treated with azacitidine; azacitidine infection risk model: The Polish Adult Leukemia Group study. Clin Lymphoma, Myeloma Leuk. 2019;19(5):264–274. e4. doi:https://doi.org/10.1016/j.clml.2019.01.002.
- Schuck A, Goette M-C, Neukirchen J, et al. A retrospective study evaluating the impact of infectious complications during azacitidine treatment. Ann Hematol. 2017;96(7):1097–1104. doi:https://doi.org/10.1007/s00277-017-3001-2.