ABSTRACT
Objectives: The objective of the study was to assess the tolerability and effectiveness of micafungin prophylaxis during the neutropenic phase in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Methods We conducted a retrospective study of 73 consecutive adults receiving antifungal prophylaxis with micafungin bridged to voriconazole/itraconazole in our center from July 2013 to March 2018. Clinical and transplant-related demographics and data on fungal infection post-transplant were collected. Results Micafungin was effective in 71 (97.3%) leukopenic patients. The fungal-free survival was 91.8%, 80.6%, and 77.6% respectively at 30, 60, and 100 days after HSCT. All patients had no micafungin-related adverse events. Conclusions The utility of micafungin bridged to voriconazole/ itraconazole for antifungal prophylaxis after HSCT is beneficial.
Introduction
Invasive fungal infections (IFIs) are an important cause of morbidity and mortality in allogeneic hematopoietic stem cell transplantation (HSCT) recipients [Citation1,Citation2]. Effective prevention of fungal infection plays a decisive role in transplant success. Several systemic antifungal agents (caspofungin, micafungin, itraconazole, fluconazole and amphotericin B, posaconazole) have been used for fungal prophylaxis. However, challenges of some antifungal agents include the high costs, adverse reactions such as severe renal toxicity and hepatotoxicity, drug–drug interactions, and limited antifungal spectrum. So the selection of an appropriate antifungal treatment is very important for HSCT patients.
Micafungin is an echinocandin antifungal agent and effective against both Candida and Aspergillus [Citation3]. Moreover, micafungin has a slight effect on cytochrome P450 (CYP450) isoenzymes. Therefore, micafungin has higher safety and less drug interaction compared to other antifungal agents such as triazoles and amphotericin B [Citation4]. At present, micafungin is strongly recommended for prophylaxis therapy in neutropenic patients receiving HSCT [Citation5–9]. The aim of this study was to assess the efficacy and safety of micafungin for antifungal prophylaxis during neutropenia after transplantation.
Material and methods
Patients
We retrospectively enrolled 73 consecutive patients with hematopoietic malignancies who received micafungin for antifungal prophylaxis during neutropenia after transplantation in the third affiliated hospital of Sun Yat-Sen University from July 2013 to March 2018. The clinical data were collected including the characteristics of patients and transplantation, underlying disease, neutrophil implantation time, time of fungal infection, history of fungal infection, galactomannan antigen from blood, and chest CT imaging results. Patients were followed up until fungal infection, death or day 100 after HSCT, whichever emerged first. We obtained informed consent from patients or the patients’ guardians. The study protocol was approved by the ethics committee of the third affiliated hospital of Sun Yat-Sen University.
Transplant regimen
The major preconditioning treatment consisted of cytarabine, busulfan, cyclophosphamide, and semustine along with rabbit anti-thymocyte globulin (ATG). For recipients receiving matched-sibling donor (MSD) transplant, cyclosporine A (CsA) and short-term methotrexate (MTX) were used for graft-versus-host disease (GVHD) prophylaxis. Patients who underwent well-matched unrelated or haploidentical HSCT received CsA, mycophenolate mofetil (MMF) and short-term MTX for GVHD prophylaxis. Umbilical cord blood transplantation (UCBT) recipients received a total body irradiation (TBI)-based conditioning regimen and their GVHD prophylaxis regimen consisted of CsA and MMF.
All of the patients from the beginning of conditioning received micafungin as antifungal prophylaxis (primary prevention 50 or 100 mg, secondary prevention 100 or 150 mg) until engraftment. After the engraftment, the patients without existing fungal infection were switched to oral voriconazole prophylaxis if having a history of fungal infection; otherwise oral itraconazole as fungal prophylaxis. Antimicrobial and antiviral prophylaxis from the beginning of the conditioning regimen was rendered. Six patients were given ganciclovir as antiviral prophylaxis, the other patients were given acyclovir. Prophylactic anti-bacterial agents mainly included quinolone and β-lactam antibiotics.
If fever persisted for more than 72 h after broad-spectrum anti-bacterial treatment was performed, inflammatory indicators, microbiological examination and imaging examination of the infected site were completed. Broad-spectrum anti-bacterial treatment referred to antibiotics against Gram-positive and Gram-negative bacteria were given. Possible, probable or proven IFI was diagnosed according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria [Citation1].
Definition
Failure of prophylaxis was considered in those patients who developed a fungal infection from transplantation preconditioning to 30 days after transplantation. Severe side effects of micafungin were defined in patients who are no longer able to continue using micafungin due to adverse reaction, otherwise, mild or moderate adverse action was defined. Fungal-free survival was defined as survival without probable or proven IFD.
Statistical analyses
Categorical variables and continuous variables were respectively compared with chi-square test and the Mann–Whitney U test. Categorical data were expressed in frequency. Continuous variables are expressed as the median and interquartile range (IQR). Kaplan-Meier curves were drawn and log-rank tests were applied to analyze the rate of fungal infection. P < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
During the study period, data from 73 patients receiving HSCT are collected. All the demographic characteristics of the patients at baseline are summarized in .
Table 1. Characteristics of the patients and HSCT.
Treatment exposure
A total of 26 patients had a history of pulmonary fungal infection. About 47 patients had no previous fungal infection. The most common dose used in our center was 100 mg/day in 36 (49.3%) patients, while 34.2% patients received 50 mg/day micafungin. The other patients used 150 mg/day micafungin.
Clinical outcome
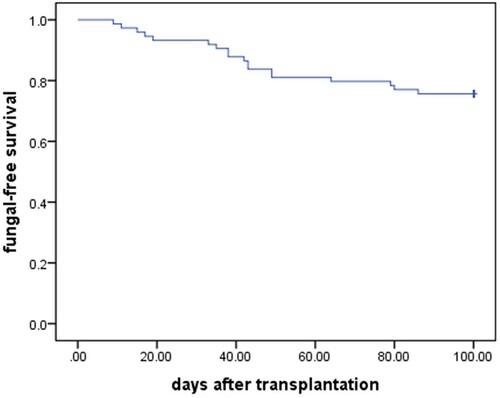
Besides two patients who developed probable IFI during neutropenia period, 71 patients received micafungin as antifungal prophylaxis until neutrophil implantation. Therefore micafungin was effective in 71 (97.3%) leukopenic patients. The clinical data of two patients with IFI are shown in . The fungal-free survival was 91.8%, 80.6%, and 77.6% respectively at 30, 60, and 100 days after HSCT ().
Table 2. Clinical data of two patients with failure of micafungin prophylaxis during neutropenia period.
Adverse reactions
All patients had no micafungin-related adverse events. Micafungin did not affect serum concentration of immunosuppressive agents.
Discussion
Due to bowel mucosal damage from cytotoxic chemotherapy, neutropenia, use of central intra-venous catheters, immunosuppressive therapy and glucocorticoid use for GVHD, allogeneic HSCT recipients are susceptible to fungal infection. As an early diagnosis of IFIs is difficult, its treatment is challenging and therapy costs are extraordinarily high, antifungal prophylaxis is important for HSCT patients [Citation10].
For many years, fluconazole has been the antifungal drug of choice for primary prevention in HSCT recipients [Citation11]. However, with the change of the epidemiological characteristics of invasive mycosis and increase in resistant yeasts, the application of fluconazole is gradually limited. In addition, triazole antifungals are mainly metabolized by liver CYP450 isoenzymes, which increases drug–drug interactions such as the serum concentration of immunosuppressive agents [Citation12]. However, other azole antifungals including itraconazole, voriconazole and posaconazole offset a broad spectrum of antifungal activity(Candida sp., Aspergillus sp. and other relatively rarer molds) against their adverse reaction. Posaconazole as a newer antifungal agent is costly and available only orally. In adult patients with hematologic malignancies, guidelines only moderately support the use of liposomal amphotericin B and provide marginal support for fluconazole [Citation13].
Representatives of the echinocandins include caspofungin and micafungin. They have a lower prevalence of treatment-related hepatic adverse reaction and nephrotoxicity compared to azoles [Citation14], but caspofungin as a prophylactic agent is limited by the necessity of i.v. infusion and has a higher cost than its similar drug-micafungin. It was considered micafungin prophylaxis was shown to be effective and safe for HSCT recipients [Citation7]. A retrospective cohort study reported 93 pediatric patients undergoing HSCT in the micafungin group had better results about the incidence and resolution of fever compared to that of caspofungin [Citation15]. A health economic evaluation showed higher costs of antifungal prophylaxis were balanced by a reduced rate of IFD and lower costs of antifungal treatment in the micafungin bridging group compared to the group of oral posaconazole [Citation8].
So in theory, both venous micafungin and oral triazoles including voriconazole or itraconazole have a higher safety profile and cost–benefit ratio. We retrospectively evaluated the effectiveness of bridging oral itraconazole/voriconazole prophylaxis with intra-venous micafungin at doses of 50, 100 or 150 mg in HSCT recipients. Related data on the use of micafungin as a bridging agent are rare. Micafungin plays an antifungal role by blocking the synthesis of 1,3-b- D-glucan, which is a major component of the cell wall of most fungal cells [Citation4]. It has a wide antifungal spectrum against candida and aspergillus [Citation16,Citation17], and low drug–drug interactions.
Early fungal infection in HSCT recipients usually occurs about one month after transplantation, mainly during the hematopoietic reconstruction period, and the fungal spectrum is dominated by aspergillus and candida. In our analysis, micafungin was shown to be an effective prophylaxis against IFI in the allo-HSCT setting. Only two patients developed breakthrough fungal infection during neutropenia. The fungal-free survival was 91.8%, 80.6%, and 77.6% respectively at 30, 60, and 100 days after HSCT. None of the patients died from IFI. Several studies demonstrated that micafungin is either similar or superior to fluconazole for prophylaxis of fungal infections among patients undergoing HSCT [Citation13,Citation18]. A meta-analysis by Lee et al. also showed that micafungin was significantly associated with higher treatment success, lower rates of IFIs, and fewer adverse reactions compared with triazoles [Citation5]. Different doses of micafungin were shown to be effective for IFIs prophylaxis in patients with hematological malignancy [Citation19].
In our study, there was no significant toxicity induced by micafungin. This is in line with previous studies including a retrospective study for patients with hematologic malignancies, in which there was no evidence that liver or renal toxicity was related to micafungin prophylaxis at doses of up to 300 mg (2–3 doses per week) [Citation20,Citation21].
Indeed, the cause of hepatotoxicity or nephrotoxicity is likely to be multifactorial in HSCT. Therefore, it was difficult to determine whether the hepatic adverse reaction was due to micafungin or other post-transplant complications, such as GVHD. A clinical trial including 3028 patients showed no clear association between higher doses of micafungin or longer treatment duration and higher rates of treatment-related adverse events [Citation22]. Overall, micafungin has low toxicity and is well tolerated in the HSCT transplant setting.
The present study had several potential limitations. First, we assume some baseline characteristics such as age, underlying condition, and the strategy for GVHD prophylaxis do not have an impact on the effectiveness of the micafungin prophylaxis. In addition, this retrospective study was not powered to show significant differences between dosage levels, because the sample size of patients in each group was small. Finally, prevention strategy could not be divided into primary prevention and secondary prevention to evaluate the efficacy of micafungin treatment.
In summary, micafungin was effective and well tolerated in clinical practice as prophylaxis in HSCT patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases Mycoses Study Group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–1821. doi:https://doi.org/10.1086/588660.
- Drgona L, Khachatryan A, Stephens J, et al. Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur J Clin Microbiol Infect Dis. 2014;33:7–21. doi:https://doi.org/10.1007/s10096-013-1944-3.
- Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46:150–156. doi:https://doi.org/10.1128/JCM.01901-07.
- Patil A, Majumdar S. Echinocandins in antifungal pharmacotherapy. J Pharm Pharmacol. 2017;69:1635–1660. doi:https://doi.org/10.1111/jphp.12780.
- Ullmann AJ, Cornely OA, Donnelly JP, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect. 2012;18(Suppl 7):1–8. doi:https://doi.org/10.1111/1469-0691.12037.
- Langebrake C, Rohde H, Lellek H, et al. Micafungin as antifungal prophylaxis in recipients of allogeneic hematopoietic stem cell transplantation: results of different dosage levels in clinical practice. Clin Transpl. 2014;28:286–291.
- Park S, Kim K, Jang JH, et al. Randomized trial of micafungin versus fluconazole as prophylaxis against invasive fungal infections in hematopoietic stem cell transplant recipients. J Inf Secur. 2016;73:496–505.
- Oyake T, Kowata S, Murai K, et al. Comparison of micafungin and voriconazole as empirical antifungal therapies in febrile neutropenic patients with hematological disorders: a randomized controlled trial. Eur J Haematol. 2016;96:602–609.
- Epstein DJ, Seo SK, Brown JM, et al. Echinocandin prophylaxis in patients undergoing haematopoietic cell transplantation and other treatments for haematological malignancies. J Antimicrob Chemother. 2018;73:i60–i72.
- Rieger CT, Ostermann H, Kolb HJ, et al. A clinical cohort trial of antifungal combination therapy: efficacy and toxicity in haematological cancer patients. Ann Hematol. 2008;87:915–922. doi:https://doi.org/10.1007/s00277-008-0534-4.
- Xu SX, Shen JL, Tang XF, et al. Newer antifungal agents micafungin and voriconazole for fungal infection prevention during hematopoietic cell transplantation: a meta-analysis. Eur Rev Med Pharmacol Sci. 2016;20:381–390. PMID: 26875911.
- Heimann SM, Vehreschild MJ, Meintker L, et al. Different doses of micafungin for prophylaxis of invasive fungal diseases in hemato-oncological high-risk patients: a web-based non-interventional trial in four large university hospitals in Germany. Transpl Infect Dis. 2014;16:968–974. doi:https://doi.org/10.1111/tid.12305.
- Cornely OA, Pappas PG, Young JA, et al. Accumulated safety data of micafungin in therapy and prophylaxis in fungal diseases. Expert Opin Drug Saf. 2011;10:171–183. doi:https://doi.org/10.1517/14740338.2011.557062.
- Hiramatsu Y, Maeda Y, Fujii N, et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2008;88:588–595. doi:https://doi.org/10.1007/s12185-008-0196-y.
- Lee CH, Lin JC, Ho CL, et al. Efficacy and safety of micafungin versus extensive azoles in the prevention and treatment of invasive fungal infections for neutropenia patients with hematological malignancies: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0180050.
- Jeong SH, Kim DY, Jang JH, et al. Efficacy and safety of micafungin versus intravenous itraconazole as empirical antifungal therapy for febrile neutropenic patients with hematological malignancies: a randomized, controlled, prospective, multicenter study. Ann Hematol. 2016;95:337–344. doi:https://doi.org/10.1007/s00277-015-2545-2.
- Wang JF, Xue Y, Zhu XB, et al. Effificacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: a meta analysis of RCTs. Eur J Clin Microbiol Infect Dis. 2015;34(4):651–659. doi:https://doi.org/10.1007/s10096-014-2287-4.
- Neofytos D, Huang YT, Cheng K, et al. Safety and effificacy of intermittent intravenous administration of high-dose micafungin. Clin Infect Dis. 2015;61(Suppl 6):S652–S661. doi:https://doi.org/10.1093/cid/civ818.
- van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39(10):1407–1416. doi:https://doi.org/10.1086/422312.
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation–a prospective randomized double blind study. J Infect Dis. 1995;171:1545–1552. doi:https://doi.org/10.1093/infdis/171.6.1545.
- Yamaguchi M, Kurokawa T, Ishiyama K, et al. Efficacy and safety of micafungin as an empirical therapy for invasive fungal infections in patients with hematologic disorders: a multicenter, prospective study. Ann Hematol. 2011;90:1209–1217. doi:https://doi.org/10.1007/s00277-011-1277-1.
- Park JS, Kim DH, Choi CW, et al. Efficacy and safety of micafungin as an empirical antifungal agent for febrile neutropenic patients with hematological diseases. Acta Haematol. 2010;124:92–97. doi:https://doi.org/10.1159/000315558.