ABSTRACT
Objectives
Considering the advances in functional rehabilitation in recent decades, therapist-oriented home rehabilitation (TOHR) has been increasingly used in the field of physical therapy because it increases patient compliance and reduces health system costs. The objective of this study was to investigate the effects of TOHR on functional capacity, muscle strength, and quality of life (QoL) in adults with sickle cell anemia (SCA).
Methods
Forty adults with SCA underwent manually guided TOHR for 12 weeks. Before and at the end of training, the following variables were assessed in the participants: distance covered in the 6-min walk test (6MWD); maximal inspiratory pressure (MIP); maximal expiratory pressure (MEP); handgrip strength (HGS); quadriceps strength (QS); and QoL using the Short Form-36 physical component summary (SF-36PCS) and the Short Form-36 mental component summary (SF-36MCS).
Results
After TOHR, significant increases were observed in the mean values for the 6MWD, MIP, MEP, HGS, QS, and SF-36PCS and SF-36MCS scores. The relative delta between the pre- and post-TOHR 6MWDs correlated significantly with the relative deltas of MIP (rs = 0.640, p < 0.0001), MEP (rs = 0.587, p < 0.0001), HGS (rs = 0.360, p = 0.022), and QS (rs = 0.351, p = 0.026). When the participants were separated according to their use of hydroxyurea, significant increases were observed in the relative deltas of the 6MWD, MIP and MEP values.
Conclusions
This study shows that TOHR can potentially increase functional capacity, muscle strength, and QoL in adults with SCA. Furthermore, there appears to be a relationship between 6MWD gains and muscle strength gains with TOHR.
Trial registration: ClinicalTrials.gov identifier: NCT04705792.
Introduction
Sickle cell anemia (SCA) is a life-threatening hereditary hemoglobinopathy characterized by hemoglobin (Hb) polymerization that affects many people worldwide [Citation1]. As a consequence of this process, intense sickling of red blood cells occurs, followed by the development of chronic hemolytic anemia, recurrent episodes of vaso-occlusive crisis (VOC), and damage to multiple organs [Citation2]. In SCA, oxygen transport to tissues is reduced due to several factors, including chronic anemia, reduced blood flow in the microvasculature, and Hb desaturation [Citation2, Citation3]. Collectively, these changes can negatively affect several organs and tissues and reduce the functional capacity and quality of life (QoL) of affected individuals. Despite the burden of the disease, the life expectancy of people with SCA has increased worldwide as a result of a deeper understanding of its pathophysiology and frequent improvements in therapeutic approaches [Citation4].
Skeletal muscle is among the tissues that suffer the most from SCA; it undergoes several structural modifications, including hypotrophy and extensive local microvascular remodeling [Citation2, Citation5, Citation6]. In SCA, a lower capillary density and reduced capillary tortuosity – 2 fundamental determinants of the oxygen supply to muscle tissue – alter the exchange surface area and thus increase the distance for oxygen diffusion [Citation7]. In addition, the skeletal muscles of individuals with SCA have a reduced oxidative capacity, which reduces the oxygen supply to these structures and decreases their ability to use oxygen for energy metabolism [Citation6]. Thus, in addition to anemia and pulmonary and peripheral vascular disease, the myopathy that typically develops in SCA patients may be an important contributor to exercise limitations in this population [Citation3].
Reduced physical capacity is common in people with SCA as a result of anemia and multiple pulmonary, cardiac, endothelial, muscular, and metabolic dysfunctions [Citation8–10]. In addition, these individuals have left ventricular diastolic dysfunction, lung parenchyma dysfunction, pulmonary vascular disease, arterial oxygen desaturation, muscle weakness, and abnormal accumulation of blood lactate, which together further worsen functional capacity [Citation3, Citation8]. Thus, complications such as chronic hemolytic anemia, episodes of recurrent pain, and acute and chronic injuries of multiple organs have a tremendous impact on the functional capacity of individuals with SCA [Citation11]. The pathophysiological contributors to exercise intolerance in SCA include oxygen supply limitations, abnormal cardiopulmonary responses, increased inflammation, and endothelial dysfunction [Citation3, Citation12]. Given these concerns, evaluating the impact of physical training on functional capacity is important in this population.
In people with SCA, regular exercise may decrease the risk of exercise-related inflammatory reactions and may increase the vasodilator reserve, which in turn may decrease the risk of VOC [Citation13]. Training with resistance exercises seems to be an effective strategy to improve the oxygen supply to muscles by increasing both capillary density and capillary tortuosity in healthy individuals and those with pathophysiological conditions, including SCA [Citation2, Citation6, Citation8, Citation14]. In fact, the hypotrophy and the change in oxidative capacity that occur in people with SCA are at least partially neutralized by physical training [Citation6]. In addition to improving inflammatory markers, evidence indicates that aerobic physical training also has a beneficial effect on the immune system in this population [Citation15]. Despite all these benefits, the concern that high-intensity exercise may trigger SCA complications such as VOC still represents a barrier to the prescription of exercise for people with the disease [Citation11].
The metabolic and structural defects observed in the skeletal muscles of individuals with SCA contribute to reduced muscle function, exercise tolerance, and QoL. Any intervention that can reverse these structural, metabolic, and functional muscle dysfunctions should be considered a potential therapeutic strategy. Given the feasibility and safety of exercise training and its potential benefits for people with SCA, exercise should be considered a possible strategy for neutralizing the microvascular defects of skeletal muscles in this population [Citation6, Citation11]. Considering the recent advances in functional rehabilitation, therapist-oriented home rehabilitation (TOHR) has been increasingly used in the field of physical therapy since it can increase patient compliance and reduce health system costs. Moreover, its results are comparable to those of the face-to-face approach [Citation16, Citation17]. To date, however, no study has evaluated the beneficial effects of TOHR on physical capacity in people with SCA. Thus, the present study aims to investigate the effects of TOHR on functional capacity, muscle strength, and QoL in adults with SCA.
Materials and methods
Setting and study design
Between November 2019 and January 2021, we evaluated 40 adults with SCA (of 47 eligible adults) who were regularly treated at the Pedro Ernesto University Hospital at Rio de Janeiro State University (Brazil). The study included only subjects with homozygous disease confirmed by Hb electrophoresis who were in a steady state. A steady state was defined as a period without VOC or acute chest syndrome (ACS) for 1 month prior to the study and the absence of blood transfusion during the 3 months preceding the study [Citation5, Citation8]. The following exclusion criteria were used: a history of stroke, a history of avascular necrosis, and nonadherence to the intervention protocol. None of the participants began treatment with hydroxyurea or changed their hydroxyurea dose less than 1 month before enrollment or during the study.
The experiment was approved by the Research Ethics Committee of the Augusto Motta University Center (Brazil) under number CAAE-09131519.6.0000.5235 and was compliant with the provisions of the Declaration of Helsinki. All individuals signed an informed consent form. This trial was registered at www.clinicaltrials.gov as #NCT04705792.
Intervention
The participants underwent a manually guided exercise program 3 times a week for 12 weeks. Each session included muscle training, aerobic resistance, and flexibility exercises and lasted approximately 60 min. The session began with 5 min of warm-up exercises, followed by 20 min of muscle strengthening and resistance exercises using light weights and functional diagonal movements of both the upper and lower limbs. Subsequently, 10 min of balance training was carried out using proprioceptive exercises on the ground, followed by 20 min of aerobic training by walking in functional circuits. Finally, 5 min of global stretching and relaxation exercises were performed using calisthenic exercises for the upper and lower limbs [Citation16, Citation18]. The patients were reassessed 12 weeks after the initial physical therapy evaluation and inclusion in the protocol, and TOHR was then completed. The physical therapist contacted the participants on a weekly basis to monitor treatment progress.
Quality of life
The Short Form-36 (SF-36) was used to evaluate QoL. This multidimensional self-reported tool consists of 36 items grouped into 8 dimensions that can be divided into 2 general components: the physical component summary (SF-36PCS) with 21 items and the mental component summary (SF-36MCS) with 14 items [Citation19]. The scores are converted into a scale ranging from 0–100 points, where higher scores indicate better QoL.
Respiratory muscle strength (RMS)
RMS was measured using an HD CPL device (nSpire Health, Inc., Longmont, CO, USA). For this test, the participant inhaled rapidly and continued inhaling for 1.5 sec to measure the maximal inspiratory pressure (MIP); then, the participant fully inhaled and then exhaled rapidly and completely against the occluded airway for 1–3 sec to measure the maximal expiratory pressure (MEP). Both MIP and MEP were measured at least 3 times, and the highest values for each measure were chosen for analysis. We used the predicted values of Neder et al. [Citation20] for interpretation of these measures.
Handgrip strength (HGS)
HGS was measured with an isometric hydraulic hand dynamometer (SH5001, Saehan Corporation, Korea) using the dominant hand following previously established recommendations [Citation21]. The participants were seated with the elbow flexed at 90° and the forearm in the neutral position and were asked to complete 3 maximum-effort measurements lasting 4–5 sec and separated by 60-sec intervals. The highest value among the 3 measurements was chosen for the analysis, and the predicted values of Neder et al. [Citation20] were used for interpretation.
Quadriceps strength (QS)
QS was measured using a traction dynamometer with a sensor capacity of 200 kg (E-lastic 5.0, E-sporte SE, Brazil). The range of motion during the test was determined starting at 90° with the knee flexed. Maximum strength was assessed after a 5-sec sustained contraction in the dominant leg. The highest value of 3 tests with 1-min intervals was considered for analysis [Citation22].
Six-minute walk test (6MWT)
The 6MWT was performed in a 30-m hallway, which was marked every 3 m with colored tape on the ground and with 2 cones for turning around according to previous recommendations [Citation23]. Before and at the end of the test, heart rate, respiratory rate, blood pressure, and the perceived dyspnea level were measured according to the Borg scale. Two tests were performed with a minimum rest interval of 30 min to avoid learning and adaptation effects, and the test with the greatest distance covered was used for the analysis. The reference values used were the Brazilian equations of Britto et al. [Citation24].
Statistical analysis
The normality of the variables was evaluated by the Shapiro–Wilk test, and the results are expressed as the mean ± SD or median (interquartile ranges) according to the Gaussian or non-Gaussian distribution of each variable. Pre- and post-TOHR measurements were compared by the Wilcoxon signed-rank test. The associations between the pre- and post-TOHR (post-TOHR value - pre-TOHR value/pre-TOHR value x 100) relative deltas of the distance covered in the 6-min walk test (6MWD) and the other measures were analyzed by the Spearman correlation coefficient (rs). The pre-TOHR measurements and relative deltas between the groups with and without hydroxyurea were compared by Student’s t-test for independent samples or Mann–Whitney test for numerical data and by Fisher’s exact test or chi-square for categorical data. Data analysis was performed using SAS 6.11 software (SAS Institute, Inc., Cary, NC, USA). Statistical significance was set at p < 0.05.
Results
Among the 47 adults with SCA who were evaluated for inclusion in the study, 7 were excluded for the following reasons: 4 due to difficulty walking (2 due to sequelae of osteoarticular lesions and 2 due to previous strokes) and 3 due to abandonment of the treatment protocol. Thus, the evaluated sample consisted of 22 women and 18 men with a mean age of 31.8 ± 14.5 years. The median Hb concentration was 8 ± 0.9 g/dL. The demographic and clinical data at baseline are shown in .
Table 1. Demographic and clinical data of the evaluated sample.
After TOHR, improvements were observed in the 6MWD, MIP, MEP, HGS, QS, and SF-36PCS and SF-36MCS scores. Thirteen patients (32.5% of the total) had a baseline 6MWD under 80% of the predicted value and improved this value above this threshold at 12 weeks. Considering the mean value of 38 kgf observed in healthy young Brazilian adults [Citation25], 8 patients (20% of the total) had a baseline HGS under 38 kgf and improved this value above this threshold at 12 weeks. The results for functional capacity, RMS, peripheral muscle strength (PMS), and QoL at baseline and after 12 weeks of exercise are shown in . No patient exhibited any adverse events during or at the end of training, including VOC and ACS.
Table 2. Functional capacity, muscle strength, and quality of life assessed pre- and post-therapist-oriented home rehabilitation.
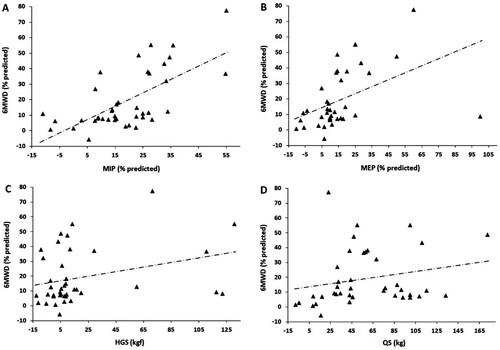
Additionally, we evaluated the correlations of the relative delta between the pre- and post-TOHR 6MWDs with the relative deltas of the other measured variables. In this analysis, the 6MWD correlated positively and significantly with MIP (rs = 0.640, p < 0.0001), MEP (rs = 0.587, p < 0.0001), HGS (rs = 0.360, p = 0.022), and QS (rs = 0.351, p = 0.026) (). No significant correlation was found between the relative deltas of the pre- and post-TOHR 6MWDs and the Hb level obtained at baseline (rs = −0.007, p = 0.96).
Figure 1. A, Relationship of the relative deltas of the 6-minute walking distance (6MWD) with the maximal inspiratory pressure (MIP, rs = 0.640, p < 0.0001), B, the maximal expiratory pressure (MEP, rs = 0.587, p < 0.0001), C, handgrip strength (HGS, rs = 0.360, p = 0.022), and D, quadriceps strength (QS, rs = 0.351, p = 0.026).
Due to a possible clinical and functional impact, we evaluated patients according to hydroxyurea use before TOHR. Regarding clinical characteristics, no significant differences in the pre-TOHR variables were observed between the 2 groups. The male/female distributions in the hydroxyurea group (11/13) and non-hydroxyurea group (7/9) showed no statistically significant difference (p = 0.90). Mean age was 30.5 ± 13.5 and 33.7 ± 16.2 for the hydroxyurea and non-hydroxyurea groups, respectively (p = 0.51). Similarly, the Hb levels between the hydroxyurea group and non-hydroxyurea group did not significantly differ (8 ± 0.9 vs. 8 ± 1 g/dL p = 0.94). The frequencies of individuals with ACS in the groups with and without hydroxyurea use were similar (45.8 vs. 43.7%, respectively, p = 0.90). No statistically significant differences between the 2 groups were observed for lung function, renal function, or tricuspid regurgitant jet velocity. Regarding functional measures (including functional capacity, muscle strength, and quality of life parameters), no significant differences in the pre-TOHR variables were observed between the 2 groups. Although the 6MWD was slightly higher in the non-hydroxyurea group, no significant difference was noted (70.6 ± 8.8 vs. 69.3 ± 12.7% predicted, p = 0.72) ().
Table 3. Absolute values and relative deltas for the measures of functional capacity, muscle strength, and quality of life assessed pre- and post-therapist-oriented home rehabilitation according to the use of hydroxyurea.
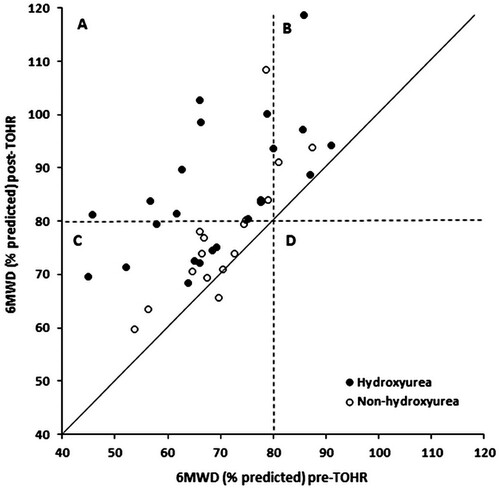
Finally, we compared pre- and post-TOHR variables according to hydroxyurea use. The absolute values and relative deltas for functional capacity, muscle strength, and quality of life according to hydroxyurea use are shown in . When the pre- and post-TOHR relative deltas for the different variables were compared, we observed significant differences for the following variables: 6MWD, MIP, and MEP. The predicted values for functional capacity according to hydroxyurea use are shown in ; almost all subjects using hydroxyurea improved with TOHR and exceeded the cutoff point of 80% predicted for the 6MWD.
Figure 2. Scatterplot showing the individual impact of therapist-oriented home rehabilitation (TOHR) on the 6-min walking distance (6MWD) according to the use of hydroxyurea. Note that (1) almost all subjects improved with TOHR (values above the main diagonal); (2) quadrant A shows that almost all subjects using hydroxyurea improved with TOHR and exceeded the cutoff point of 80% predicted for the 6MWD; and (3) quadrant C shows a predominance of subjects without hydroxyurea who improved their 6MWD but did not reach the 80% predicted cutoff point.
Discussion
The main findings of the present study were that in adults with SCA, TOHR improves performance on the 6MWT, increases respiratory and peripheral muscle strength, and improves QoL. In these patients, HGS, QS, and RMS were significantly associated with the 6MWD. In addition, hydroxyurea use seems to positively impact the effects of TOHR in this patient population, especially in terms of the 6MWD and RMS. Thus, TOHR seems to be an important rehabilitative intervention strategy for improving functional capacity, muscle function, and health-related QoL in individuals with SCA, and its incorporation into functional rehabilitation protocols for these individuals should be considered.
For patients with SCA, training, especially at exercise intensities close to those required for daily activities, improves functional capacity by reducing oxidative stress and endothelial activation [Citation8, Citation26]. In fact, we observed an increase in the 6MWD after TOHR in terms of both the absolute value and the percentage of the predicted value. The increase in the mean absolute value of the 6MWD was above the minimal clinically important difference (MCID) for adults with pathological conditions proposed in a recent systematic review, which was 30.5 m [Citation27]. Importantly, we also observed that the mean 6MWD value (predicted percentage) was <80% at baseline and increased to >80% after TOHR. In contrast, Liem et al. [Citation11] evaluated 13 individuals with SCA who underwent 3 exercise sessions/week for 12 weeks on a stationary bicycle at home and did not observe any significant improvement in the peak maximal oxygen consumption or in other exercise parameters at the end of training. According to these authors, a possible explanation for their negative results was reduced adherence to training in the second half of the program.
Exercise training can improve the muscle capillary network and partially reverse the microvascular damage observed in the skeletal muscles of patients with SCA [Citation2]. Similar to the studies by Merlet et al. [Citation2] and Camcıoğlu et al. [Citation12], we also observed an increase in muscle performance, both respiratory and peripheral, after TOHR. Several factors have been implicated in the increase in muscle function after SCA exercises, including increases in the numbers of capillaries around muscle fibers, the functional exchange surface between microvessels and muscle tissues, and the oxidative capacity of muscle tissue, especially type I fibers [Citation2, Citation12]. Interestingly, we also noted an increase in QoL assessed with the SF-36, with an MCID of at least 7 points between the mean values on the SF-36PCS and SF-36MCS when the pre- and post-TOHR evaluations were compared [Citation28]. Together, these findings suggest that exercise training is extremely important for the functional fitness of individuals with SCA in terms of their daily lives, well-being, and QoL.
The rearrangement of the microvascular network induced by capillary growth resulting from training may contribute to the concomitant improvement in functional capacity in people with SCA [Citation2]. In this regard, we observed associations between the 6MWD and muscle strength measurements, although the highest correlations were observed between the 6MWD and MIP and between the 6MWD and MEP. Similarly, Camcıoğlu et al. [Citation12] previously demonstrated the beneficial effects of inspiratory muscle training on functional capacity during exercise and on RMS in patients with SCA and recurrent ACS. Inspiratory muscle training, which was included in our protocol, improves the metaboreflex of the respiratory muscle by directing adequate blood flow to the peripheral muscles, indicating a close relationship between RMS and PMS [Citation29].
Hydroxyurea has profoundly changed the treatment of individuals with SCA. In addition to increasing the levels of fetal Hb in red blood cells and thus reducing VOCs, pain events, and hemolysis, this drug can also improve the deformability of red blood cells and change the biology of the vascular endothelium [Citation3, Citation30]. Although we observed an improvement in all parameters with TOHR in the group that used hydroxyurea compared with the group that did not use it, significant differences were noted only for the 6MWD and RMS. In agreement with our results, Hackney et al. [Citation31] observed an improvement in anaerobic performance in patients treated with hydroxyurea: these individuals had a 2-fold increase in maximum anaerobic power compared to individuals who did not use hydroxyurea. These authors also observed that aerobic performance increased in the group treated with hydroxyurea, which showed a decrease in the heart rate response after treatment for a given submaximal exercise intensity.
Notably, none of our patients had any complications during or at the end of training, including VOC or ACS. Using an 8-week moderate-intensity resistance training program on a cycle ergometer in adults with SCA, Gellen et al. [Citation8] also found no complications that required hospital admission. These findings are important because, as strenuous exercises can potentially trigger VOCs, many individuals with SCA are often advised to avoid physical activities and thus adopt a sedentary lifestyle. This approach can lead to deconditioning, which in turn aggravates exercise intolerance and results in a sedentary lifestyle and worsening of QoL.
The strength of this study is that it showed the positive results of a home-based, manually guided physical rehabilitation program for people with SCA, which may increase adherence to treatment. However, some limitations should be noted. First, the small sample size and the nonrandomized and open-label nature of the trial may limit the generalizability of our findings. Second, the evaluation of the impact of TOHR was performed by physicians who were not blinded to the study time point (baseline or post-TOHR), introducing a potential source of bias. Third, the exercise program was not conducted in person, which makes it difficult to supervise the activities recommended by the protocol. Fourth, 40% of the patients did not use hydroxyurea, which hinders a more reliable evaluation of the effect of treatment with this drug. Further studies with a larger number of individuals and randomized studies are needed to explore the impact of exercise training at home and the long-term effects among individuals with SCA.
In conclusion, this study shows that in adults with SCA, TOHR can potentially increase functional capacity, muscle strength, and QoL. There appears to be a relationship between the distance covered in the 6MWT and muscle strength. In addition, hydroxyurea treatment appears to improve the effects of TOHR in this patient population. Thus, our results can serve as a starting point for considering home training as a therapeutic strategy for adults with SCA. Since exercise training is a therapeutic option for people with SCA, exercise modalities should be evaluated for possible inclusion in educational programs for this population.
Availability of data and materials
The dataset used and analyzed during the current study is available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ware RE, de Montalembert M, Tshilolo L, et al. Sickle cell disease. Lancet. 2017;390(10091):311–323.
- Merlet AN, Messonnier LA, Coudy-Gandilhon C, et al. Beneficial effects of endurance exercise training on skeletal muscle microvasculature in sickle cell disease patients. Blood. 2019;134(25):2233–2241.
- Connes P, Machado R, Hue O. Exercise limitation, exercise testing and exercise recommendations in sickle cell anemia. Clin Hemorheol Microcirc. 2011;49(1–4):151–163.
- Pecker LH, Lanzkron S. Sickle cell disease. Ann Intern Med. 2021;174(1):ITC1–ITC16.
- Gonçalves CEA, Silva PO, Soares MS, et al. Muscle dysfunction is associated with poorer health-related quality of life in adults with sickle cell anaemia. J Back Musculoskelet Rehabil. 2019;32(1):43–53.
- Merlet AN, Féasson L, Bartolucci P, et al. Muscle structural, energetic and functional benefits of endurance exercise training in sickle cell disease. Am J Hematol. 2020;95(11):1257–1268.
- Ravelojaona M, Féasson L, Oyono-Enguéllé S, et al. Evidence for a profound remodeling of skeletal muscle and its microvasculature in sickle cell anemia. Am J Pathol. 2015;185(5):1448–1456.
- Gellen B, Messonnier LA, Galactéros F, et al. Moderate-intensity endurance-exercise training in patients with sickle-cell disease without severe chronic complications (EXDRE): an open-label randomised controlled trial. Lancet Haematol. 2018;5(11):e554–e562.
- Lopes AJ, Marinho CL, Alves UD, et al. Relationship between ventilation heterogeneity and exercise intolerance in adults with sickle cell anemia. Braz J Med Biol Res. 2017;50(8):e6512.
- Marinho CL, Maioli MC, Soares AR, et al. Predictive models of six-minute walking distance in adults with sickle cell anemia: implications for rehabilitation. J Bodyw Mov Ther. 2016;20(4):824–831.
- Liem RI, Akinosun M, Muntz DS, et al. Feasibility and safety of home exercise training in children with sickle cell anemia. Pediatr Blood Cancer. 2017;64(12):1–4.
- Camcıoğlu B, Boşnak-Güçlü M, Karadalli MN, et al. The role of inspiratory muscle training in sickle cell anemia related pulmonary damage due to recurrent acute chest syndrome attacks. Case Rep Hematol. 2015;2015:780159.
- Barbeau P, Woods KF, Ramsey LT, et al. Exercise in sickle cell anemia: effect on inflammatory and vasoactive mediators. Endothelium. 2001;8(2):147–155.
- Baum O, Gübeli J, Frese S, et al. Angiogenesis-related ultrastructural changes to capillaries in human skeletal muscle in response to endurance exercise. J Appl Physiol. 2015;119(10):1118–1126.
- El-Kader SMA, Al-Shreef FM. Impact of aerobic exercises on selected inflammatory markers and immune system response among patients with sickle cell anemia in asymptomatic steady state. Afr Health Sci. 2018;18(1):111–119.
- Lima TRL, Kasuki L, Gadelha M, et al. Physical exercise improves functional capacity and quality of life in patients with acromegaly: a 12-week follow-up study. Endocrine. 2019;66(2):301–309.
- Kraal JJ, Van den Akker-Van Marle ME, Abu-Hanna A, et al. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: results of the FIT@Home study. Eur J Prev Cardiol. 2017;24(12):1260–1273.
- Baltich J, Emery CA, Stefanyshyn D, et al. The effects of isolated ankle strengthening and functional balance training on strength, running mechanics, postural control and injury prevention in novice runners: design of a randomized controlled trial. BMC Musculoskelet Disord. 2014;15:407.
- Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J. 1992;305(6846):160–164.
- Neder JA, Andreoni S, Lerario MC, et al. Reference values for lung function tests. II. maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719–727.
- Crosby CA, Wehbe MA, Mawr B. Hand strength: normative values. J Hand Surg Am. 1994;19(4):665–670.
- Andrade Junior AB, Ferreira AS, Assis ACB, et al. Cardiac autonomic control in women with rheumatoid arthritis during the glittre activities of daily living test. Asian J Sports Med. 2020;11(2):e101400.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Britto RR, Probst VS, de Andrade AF, et al. Reference equations for the six-minute walk distance based on a Brazilian multicenter study. Braz J Phys Ther. 2013;17(6):556–563.
- Neves RS, Lopes AJ, Menezes SLS, et al. Hand grip strength in healthy young and older Brazilian adults: development of a linear prediction model using simple anthropometric variables. Kinesiology. 2017;49(2):208–216.
- Charrin E, Aufradet E, Douillard A, et al. Oxidative stress is decreased in physically active sickle cell SAD mice. Br J Haematol. 2015;168(5):747–756.
- Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381.
- Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower. Arthritis Rheum. 2001;45(4):384–391.
- Waltz X, Hedreville M, Sinnapah S, et al. Delayed beneficial effect of acute exercise on red blood cell aggregate strength in patients with sickle cell anemia. Clin Hemorheol Microcirc. 2012;52(1):15–26.
- Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;4(4):CD002202.
- Hackney AC, Hezier W, Gulledge TP, et al. Effects of hydroxyurea administration on the body weight, body composition and exercise performance of patients with sickle-cell anaemia. Clin Sci. 1997;92(5):481–486.
