ABSTRACT
Objectives
Myeloma relapse remains challenging. Daratumumab (dara) with immunomodulatory agents (IMiD) and dexamethasone (dex) was proven highly effective in relapsed or refractory multiple myeloma (RRMM) in randomized controlled trials. The recommended schedule of dara is weekly for eight doses, followed by 2-weekly for eight doses, and then every 4-weekly thereafter. However, the cost of daratumumab is daunting, precluding widespread and prolonged use in some countries. In this study, we aimed to evaluate the efficacy of using a 3-weekly daratumumab regimen in RRMM.
Methods
Thirteen RRMM patients were treated with dara-IMiD-dex till maximal response, followed by single-agent IMiD maintenance until disease progression. Dara (every 6 weekly) would be added upon significant biochemical disease progression.
Results
After a median of four daratumumab infusions (range: 3–10), the best responses included complete response (CR) in seven patients (53.8%), very good partial response (VGPR) in four patients (30.8%), and partial response (PR) in two patients (15.4%). The median time to VGPR was four weeks. At 10 months, the overall survival was 90%, and progression-free survival was 54.7%. Two of three patients tested achieved MRD-ve CR. Another patient, who had PET-CT reassessment, showed PET-ve CR.
Discussion
Despite less frequent daratumumab use, we reported rapid responses with a median time to VGPR of only four weeks, and a response rate of 100% including CR rate of 54%. Despite less frequent daratumumab use, grade ¾ neutropenia remained common with a frequency comparable to that observed in Pollux.
Conclusion
This 3-weekly dara-IMiD-dex regimen preserves a high efficacy with rapid, deep responses including MRD-ve and PET-ve CR, hence a cost-effective regimen.
Introduction
Despite a much-improved survival with bortezomib-based induction, myeloma relapse remains challenging [Citation1,Citation2]. Daratumumab is a fully human IgGK monoclonal antibody targeting CD38 [Citation3]. In a randomized controlled trial, Pollux, in which myeloma patients in early relapse were randomized to receive lenalidomide/dexamethasone with or without daratumumab, patients receiving daratumumab yielded superior response rates (response rate [RR] of 93%, ≥complete response: 43%), survivals and a favorable side-effect profile [Citation4]. The recommended dosing of daratumumab is 16 mg/kg weekly for 8 doses, followed by every 2-weekly for another 8 doses, and then every 4-weekly until disease progression [Citation4].
As daratumumab is an antibody with a long plasma half-life and shown to synergize with immunomodulatory agent (IMiD), less frequent dosing is potentially possible, especially when used in combination [Citation5]. Moreover, the cost of daratumumab is daunting, precluding widespread and prolonged use.
On the other hand, reimbursement of expensive novel drugs is typically slow in resource-constrained regions including Hong Kong. For instance, bortezomib was approved by FDA in 2005 but was only reimbursed by the Samaritan Fund of the Hong Kong Hospital Authority in October 2010. In Hong Kong, daratumumab is not yet reimbursed for MM. Therefore, outside clinical trials, daratumumab is available only to patients who can afford them. Based on the success of 3-weekly rituximab in the R-CHOP regimen for lymphoma, herein, we reported a 3-weekly daratumumab regimen in relapsed myeloma patients [Citation6].
Methods
Thirteen patients with relapsed or refractory MM with measurable disease (defined as having at least one of the following: serum M-protein ≥0.5 g/dL, urine M-protein ≥200 mg/24 h, involved serum free light chain ≥5 mg/dL and abnormal serum free light-chain ratio [<0.26 or >1.65], or plasmacytoma confirmed with biopsy) were enrolled. Refractory disease was defined as disease progression during or within 60 days of completion of the last therapy.
Daratumumab (16 mg/kg intravenously) was used in combination with lenalidomide/dexamethasone (DRd) or pomalidomide/dexamethasone (DPd) in patients who were lenalidomide-refractory. The dose of lenalidomide was 25mg/day (5 days per week), and in patients refractory to lenalidomide, the dose of pomalidomide was 4 mg/day (5 days per week). The dose of dexamethasone was 40 mg/week [Citation2]. Patient achieving sustained maximal response, documented by two tests at least 4 weeks apart, received single-agent IMiD maintenance until disease progression. Therefore, lenalidomide would be continued in those receiving DRd induction, and pomalidomide in those receiving DPd. Daratumumab (16 mg/kg every 6-weekly) would be added as a maintenance upon significant biochemical disease progression with M-protein increased by 25%, and measured >5 g/L or urine M-protein >200 mg/24 hr, or involved serum free light chain ≥10 mg/dL with abnormal serum free light-chain ratio. Minimal residual disease (MRD) testing was done by ASO-RQPCR as reported [Citation7].
This study was approved by the Institutional Review Board of the University of Hong Kong / Hospital Authority Hong Kong West Cluster (Reference number UW 20-747) and written informed consents had been obtained from patients.
Statistics
Responses were defined according to the IMWG response criteria. Overall survival (OS) was defined as the time from commencement of induction therapy to death or last follow-up. Progression-free survival (PFS) was defined according to the IMWG criteria [Citation8]. Patients were clinically staged according to the international staging system (ISS) [Citation9]. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test using Statistical Package for the Social Sciences (SPSS) version 16.0. All p-values were two-sided. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03.
Results
Thirteen relapsed myeloma patients, with a median age of 63 years (range: 50–84 years), who could afford daratumumab (dara) were treated with dara-IMiD-dexamethasone till maximal response, followed by single-agent IMiD maintenance. Apart from 3 patients, in whom FISH was not available, none of the 10 with FISH data had high-risk karyotypes including t(4;14), t(14;16) or del(17p). However, 5 (50%) had 1q21 amplification. The median number of previous therapies was two (range: 1–5), with nine patients (69.2%) having undergone autologous stem cell transplantation. Three patients (23.1%) were refractory to bortezomib, seven patients (53.8%) to lenalidomide, and eight patients (61.5%) to last treatment. Three (23.1%) had extramedullary disease, two with extra-osseous sternal plasmacytoma and one with hematogenous plasmacytoma in liver. Treatment included dara-lenalidomide-dex in six (46.2%), and dara-pomalidomide-dex in seven (53.8%).
After one cycle, there were one (7.7%) complete response (CR), three (23.1%) very good partial response (VGPR), and seven (53.8%) partial response (PR). After four cycles, there were five CR (38.5%), five VGPR (38.5%), and three PR (23%). After a median of four daratumumab infusions (range: 3–10), the best responses included CR in seven patients (53.8%), VGPR in four patients (30.8%), and PR in two patients (15.4%). The median time to VGPR was 4 weeks. ((A)) At 10 months, the OS was 90%, and PFS was 54.7%. ((B)) Individual patients’ responses and outcomes were shown in the swimmer plot. ((C)) One patient achieved PET-ve CR at serological CR. () Three had MRD testing at serological CR by ASO RQPCR with a sensitivity of 10−5 that showed MRD-negativity in two but the presence of MRD was defined as MRD positive but non-quantifiable [Citation7]. Three patients progressed, one of whom died of ruptured hepatic plasmacytoma.
Figure 1. (A) Kaplan-Meier plot of time to VGPR; (B) Progression-free survival (PFS) and overall survival (OS) for RRMM treated with 3-weekly dara-IMiD-dex regimen. (C) Swimmer plot of patients’ outcome. CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease; PD: progressive disease.
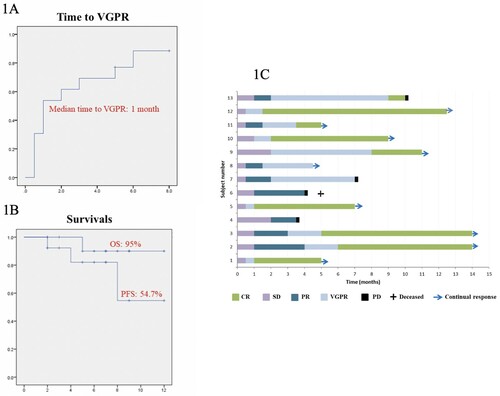
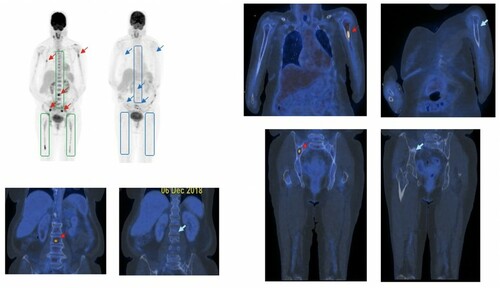
Figure 2. PET-ve CR based on the complete resolution of (i) diffuse hypermetabolic bone marrow lesions in the axial skeleton and both femurs (boxed), and (ii) focal hypermetabolic marrow lesions (red arrows) in the lumbar spine, L humerus and R iliac bone, at the time of serological CR (blue arrows).
The most frequent toxicity was haematological especially neutropenia (all grades: 92.3%, Grade ¾: 76.9%), infusion reaction (38.5%, all grade ½), neuropathy (38.5%, all grade ½), gastrointestinal (all grades: 38.5%, grade ¾: 7.7%), and sepsis (all grades: 30.8%; grade ¾: 23.1%). Neutropenia was effectively prevented with prophylactic G-CSF ().
Table 1. Treatment-emergent adverse events.
Discussions
Despite less frequent daratumumab use, we reported rapid responses with a median time to VGPR of only four weeks, and a response rate of 100% including CR rate of 54%. Albeit a small number of cases, the response rate, CR rate and speed of response compare favorably to that of Pollux [Citation4]. Two patients with sternal plasmacytomas achieved CR rapidly. The oldest patient was 84, who was considered refractory elsewhere, achieved CR readily. Given the 3-weekly usage instead of weekly for eight followed by 2-weekly for eight cycles, this de-escalated regimen is as effective but less expensive, hence more applicable in less affluent countries.
Notably, despite less frequent daratumumab use, grade ¾ neutropenia remained common with a frequency comparable to that observed in Pollux [Citation4]. Therefore, one might be cautious about potentially more frequent grade ¾ neutropenia and infections if weekly followed by every 2-weekly daratumumab was used as recommended. Herein, single-agent IMiD was used as maintenance with daratumumab added upon significant biochemical progression, hence obviating frequent hospital visits with potentially fewer side effects.
Finally, though only performed in a small number of patients, MRD-ve CR was achieved in two of the three tested by ASO-RQPCR [Citation7]. Further analysis of MRD by NGS is warranted [Citation10,Citation11]. Moreover, one patient achieved PET-ve CR during serological CR. Both MRD-ve CR and PET-ve CR have been shown important prognostic factors predicting superior PFS [Citation12,Citation13]. Hence, this 3-weekly daratumumab regimen is a highly efficacious and cost-effective regimen.
Conclusion
This 3-weekly dara-IMiD-dex regimen preserves a high efficacy with rapid, deep responses including MRD-ve and PET-ve CR, hence a cost-effective regimen. However, the frequent G3/4 neutropenia despite reduced daratumumab use warrants further studies.
Disclosure statement
The abstract of this paper was presented at the European Hematology Association 2019 as a poster presentation and was published in the abstract book of the Journal of Clinical Oncology under American Society of Clinical Oncology: https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.e19521
References
- Chim CS, Lie AK, Chan EY, et al. Treatment outcome and prognostic factor analysis in transplant-eligible Chinese myeloma patients receiving bortezomib-based induction regimens including the staged approach, PAD or VTD. J Hematol Oncol. 2012;5:28.
- Chim CS, Kumar SK, Orlowski RZ, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32:252–262.
- van de Donk NW, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;64:939–948.
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331.
- Nijhof IS, Groen RW, Noort WA, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21:2802–2810.
- Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Eng J Med. 2012;366:2008–2016.
- Bai Y, Wong KY, Fung TK, et al. High applicability of ASO-RQPCR for detection of minimal residual disease in multiple myeloma by entirely patient-specific primers/probes. J Hematol Oncol. 2016;9:107.
- Kumar S, Paiva B, Anderson KC, et al. International myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346.
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420.
- Yao Q, Bai Y, Orfao A, et al. Standardized minimal residual disease detection by next-generation sequencing in multiple myeloma. Front Oncol. 2019;9:449.
- Yao Q, Bai Y, Orfao A, et al. Upgraded standardized minimal residual disease detection by next-generation sequencing in multiple myeloma. J Mol Diagn. 2020;22(5):679–684.
- Bai Y, Orfao A, Chim CS. Molecular detection of minimal residual disease in multiple myeloma. Br J Haematol. 2018;181:11–26.
- Bartel TB, Haessler J, Brown TL, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114(10):2068–2076.
