ABSTRACT
Donor cell leukemia (DCL) or Donor cell myelodysplastic syndrome (DC-MDS) is a rare type of leukemia or MDS and originates from the donor cells. It has very low frequency, but it seems to be steadily increasing as the physiopathology and mechanisms remain largely unknown. Here we report one case of acute lymphocytic leukemia (ALL) and one case of MDS of donor origin after allogeneic hematopoietic stem cell transplantation (HSCT). We also collated and reviewed literatures regarding DCL, and discuss the morbidity of DCL, the methods used to confirm donor origin and the etiology and pathogenesis of DCL. A new proposed theory will help us to better understand the mechanism for the oncogenesis of DCL.
Dear Editor,
Acute leukemia or myelodysplastic syndrome (MDS) can develop de novo in engrafted cells of donor origin, named donor cell leukemia (DCL) or donor cell-derived MDS (DC-MDS). DCL is a rare phenomenon after allogeneic hematopoietic stem cell transplantation (allo-HSCT) since its first report in 1971. Emerging evidence suggests that DCL may account for 5% of all post-transplant leukemia relapses, even higher after cord blood transplants [Citation1, Citation2]. Here we reported one case of acute lymphocytic leukemia (ALL) and one case of MDS of donor origin after allo-HSCT from a total of 1650 patients who received HSCT between January 2000 and September 2020 from our hospital and followed standard ethical procedures (Supplementary information).
The first case was a 37-year-old male with blood type O, Rh+, diagnosed with normal karyotype B-ALL in April 2018. He achieved complete remission (CR) with two courses of VDCP regimen (Vinorelbine, daunorubicin, prednisone, and cyclophosphamide). After conditioning with the Bu/CY + ATG scheme, the patient underwent allo-HSCT from his HLA-haploidentical 13-year-old son with blood type O, Rh+. The donor’s cell engraftment was confirmed by chimeric exam and patient remained in CR after transplantation without graft versus host disease (GvHD). One year later in April 2019, patient developed unknown-cause epistaxis. Bone marrow aspirate showed lymphoblast with 31.2%. Flow cytometric analysis showed that 96% of the lymphoblast with a phenotype of CD45dim+ CD19+ CD10+ CD34+ CD38- CD20- CD58- CD123-. Karyotype analysis showed 46, XY,t(9;22)(q34;q11). Molecular analysis showed BCR/ABL T315I mutation. Repeated test of the lymphocytic blast cells from patient with 21 HLA microsatellite markers, with polymerase chain reaction (PCR) for short tandem repeats (STR), showed acomplete donor chimerism. Additionally, the molecular analysis from the donor samples showed no BCR/ABL T315I mutation before and after donation. Based on these testing results, the patient was diagnosed with DCL-ALL. After one course of chemotherapy with tyrosine kinase inhibitor Ponatinib, the patient achieved CR and remained in CR for 10 months. The donor is still healthy 2 years after donation.
The second case was a 46-year-old male with blood type AB, Rh+, diagnosed with myelodysplastic syndrome (MDS-RAEB II) 15 years ago (December 2005) with normal karyotype. He achieved CR with one course of CAG regimen chemotherapy, followed by two more courses of CAG regimen, and then underwent allo-HSCT from his HLA-haploidentical 38-year-old brother with blood type A, Rh+. Improved Bu/CY + ATG scheme was used for conditioning before HSCT. The donor’s cell engraftment was confirmed by chimeric exam and patient remained in CR after transplantation without GvHD. Thirteen years later after HSCT in August 2019, routine follow-up CBC result showed platelet 68 × 10*9/L and bone marrow aspirate showed 5.5% blast cells. The chromosome exam showed normal karyotype. The next-generation sequencing (NGS) showed DNMT3A+, TET2+ mutation. Flow cytometric results showed 5.43% abnormal blasts. Among the blasts, 80.35% of the cells have the phenotype of CD34-CD117+CD13+ CD33str+ HLA-DRpart+ CD11b-CD16-, consistent with abnormal early myeloid cells. Repeated test of the blast cells from the patient, with 21 HLA microsatellite markers with PCR for STR, showed a complete donor chimerism. Repeated analysis of the bone marrow samples from both donor and patient showed identical molecular HLA profile, suggesting that this abnormal MDS emerged from donor cells. The patient was diagnosed with DC-MDS-EB II, according to the WHO diagnosis criteria, and given two courses of Decitabine + CAG regimen, followed by one course of Azacytidine without CR. Then the patient achieved CR with one course of HAA (Harringtonine, Ara-C, Aclacinomycin) regimen. The patient remained in CR for 9 months with Venetoclax plus Ara-C consolidation therapy. The donor’s samples, at the time of graft, have been destroyed, thus no retrospective molecular analyses could be performed. Donor brother has not developed MDS or leukemia 13 years after donation.
By searching the PubMed database, we have identified a total of 97 cases reported since 1971 (Supplementary Table 1). According to the data, the DCL frequency has accelerated from 2004 (Supplementary Figure 1), with the latent period from a couple of months to more than 10 years (Supplementary Figure 2). Among the different types of DCLs reported, AML and ALL are the most common ones (Supplementary Figure 3). The spike of incidence reported recently may be largely due to the improvement in detection methods with molecular analysis and cytogenetic advances. Even with the increase of the incidence reported recently, researchers still believed that there were the underestimated cases than the actual cases and suggested that improved molecular testing would allow precise detection and avoid mistakenly categorizing DCL as a relapsed disease [Citation2, Citation3].
Several detection strategies, including conventional cytogenetics and fluorescent in situ hybridization (FISH), variable number of tandem repeats (VNTRs) and STR polymorphism, as well as whole-exome sequencing, have been used [Citation3]. The most commonly used methods for the DCL diagnosis and proofs of donor origin are STR and FISH, which are recommended by the European Society for Blood and Marrow Transplantation as the gold standard for chimerism analysis especially for the verification of DCL. However, new technologies, such as NGS and single cell sequence, could identify some newer diallelic polymorphic markers for more sensitive detection of mixed chimerism. NGS can help identify molecular markers suitable for the early detection of gene mutation, monitoring of molecular minimal residue disease, as well as for tracking of distinct mutational patterns [Citation4, Citation5], and may decipher the mechanisms of leukemogenesis in the near future.
Donor selection needs to be more careful due to the increased popularity of non-myeloblative HSCT and age of the potential donors. The management of DCL poses ethical dilemmas regarding donor notification and put the potential concerns over the future health of the donor. DCL also provides researchers a unique human model for studying the mechanisms of leukemogenesis in vivo due to its special occurrence. The latency of the DCL onset may relate to multifactorial processes including HSCs and microenvironment involving different stages of the mechanism or the impact by one or more different impact factors for the oncogenesis of DCL.
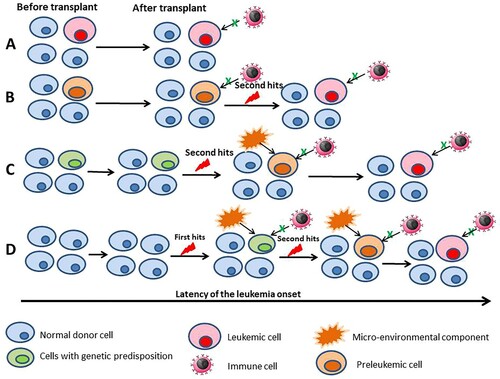
The etiology of the DCL still largely remains unknown. Several putative theories have been proposed with a multifactorial process influencing differently both the donor and the recipient, including occult leukemia in the donor, malignant predisposition of the donor cells, transfection and transformation of donor cells by host DNA either genomic or viral, impaired host immune surveillance, drug toxicity and a leukemogenic milieu in the host [Citation6]. A multiple ‘hit’ hypothesis appears plausible and a new theory () has been proposed by us, after reviewing related publications regarding the patterns of molecular pathogenesis of the DCL [Citation3, Citation7–9].
Figure 1. Possible modes of leukemia development in donor cell leukemia. (A) Established leukemic cells are transplanted and the immune surveillance failure. (B) Donor cells acquire the primary mutation before the transplant and the transplanted preleukemic cells acquire the secondary mutations; and the immune cells fail the immune surveillance. (C) Donor cells harbor a genetic predisposition and acquire the secondary mutation after the transplant; micro-environmental and/or the immune surveillance failure. (D) Donor cells after the transplant acquire the primary mutation, acquire the secondary mutation; micro-environmental and/or the immune surveillance failure. The latency period depends on the different modes and the multifactorial processes.
Although DCL or DC-MDS is a rare disease, it has to be diagnosed early. The prognosis of DCL is usually extremely poor, consistent with other secondary leukemias with a median of 5.5-month OS [Citation2, Citation3, Citation10, Citation11]. Two cases from our observation remained in CR for 12 and 8 months. Besides the traditional chemotherapy, a second HSCT from an alternative donor would remain a more logical strategy in DCL [Citation3]. Additionally, novel therapy strategies, such as molecular targeted agents [Citation12], bispecific T-cell engager antibodies, antibody–drug conjugates and chimeric antigen receptor engineered T cells (CAR-T), can be the choice for the DCL treatment in the future.
| Abbreviations | ||
| AL | = | Acute leukemia |
| ALL | = | Acute lymphocytic leukemia |
| AML | = | Acute myeloid leukemia |
| ARCH | = | Age-related clonal hematopoiesis |
| BMM | = | Bone marrow microenvironment |
| CAR-T | = | Chimeric antigen receptor engineered T cells |
| CR | = | Complete remission |
| DCL | = | Donor cell leukemia |
| DC-MDS | = | Donor cell derived myelodysplastic syndrome |
| FISH | = | Fluorescent in situ hybridization |
| GvHD | = | Graft versus host disease |
| GVL | = | Graft-versus-leukemia |
| HSC | = | Hematopoietic stem cell |
| HSCT | = | Hematopoietic stem cell transplantation |
| MDM | = | Multiple driver mutations |
| MDS | = | Myelodysplastic syndrome |
| MRD | = | Minimal residue disease |
| NGS | = | Next-generation sequence |
| PCR | = | Polymerase chain reaction |
| RDW | = | Red blood cell distribution width |
| sAML | = | Secondary acute myeloid leukemia |
| STR | = | Short tandem repeats |
| t-AML | = | Therapy-related AML |
| t-MN | = | Therapy-related myeloid neoplasms |
| UCBT | = | Umbilical cord blood transplant |
| VAF | = | Variant allele frequency |
| VNTR | = | Variable number of tandem repeats |
Supplemental Material
Download MS Word (156.5 KB)Supplemental Material
Download MS Word (98.5 KB)Acknowledgements
We thank Dr. Peter Y.Z. Jiang of Department of Hematology and Oncology, The Everett Clinic and Providence Regional Cancer Partnership in Everett, WA 98201, U.S.A. for the helpful discussion. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and based on the ethical principles for medical research involving human subjects of the Helsinki Declaration. Informed consent was obtained from all patients or legal guardians. The donor stem cells were obtained from patients’ relatives with informed consent by the medical staffs from the First Affiliated Hospital of Zhengzhou University and no organs/tissues were procured from prisoners. All authors read and approved the final manuscript for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hertenstein B, Hambach L, Bacigalupo A, et al. Development of leukemia in donor cells after allogeneic stem cell transplantation – a survey of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica. 2005;90:969–975.
- Hayes C, Petersen B, Malone A. Donor cell myeloid sarcoma in an umbilical cord transplant patient: A case report and a review of the literature. Case Rep Hematol 2015;2015:186869.
- Wiseman DH. Donor cell leukemia: a review. Biol Blood Marrow Transplant. 2011;17:771–789.
- Flach J, Shumilov E, Wiedemann G, et al. Clinical potential of introducing next-generation sequencing in patients at relapse of acute myeloid leukemia. Hematol Oncol. 2020;38(4):425–431.
- Yu J, Li Y, Zhang D, et al. Clinical implications of recurrent gene mutations in acute myeloid leukemia. Exp Hematol Oncol. 2020;9:4.
- Winer ES. Secondary acute myeloid leukemia: a primary challenge of diagnosis and treatment. Hematol Oncol Clin North Am. 2020;34:449–463.
- Taniguchi R, Muramatsu H, Okuno Y, et al. Comprehensive genetic analysis of donor cell derived leukemia with KMT2A rearrangement. Pediatr Blood Cancer. 2018;65(2).
- Bouvier A, Ribourtout B, Francois S, et al. Donor cell-derived acute promyelocytic leukemia after allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2018;101:570–574.
- Suárez-González J, Martínez-Laperche C, Martínez N, et al. Whole-exome sequencing reveals acquisition of mutations leading to the onset of donor cell leukemia after hematopoietic transplantation: a model of leukemogenesis. Leukemia. 2018;32:1822–1826.
- Suarez-Gonzalez J, Martinez-Laperche C, Kwon M, et al. Donor cell-derived hematologic neoplasms after hematopoietic stem cell transplantation: a systematic review. Biol Blood Marrow Transplant. 2018;24:1505–1513.
- Gabay T S, Chapal-Ilani N, Moskovitz Y, et al. Donor cell leukemia: reappearance of gene mutations in donor cells - more than an incidental phenomenon? Haematologica. 2020;105(12):2861–2863.
- Yu J, Jiang PYZ, Sun H, et al. Advances in targeted therapy for acute myeloid leukemia. Biomark Res. 2020;8:17.
