ABSTRACT
Objectives
This study investigates efficacy of decitabine and priming regimen in treating newly diagnosed acute myeloid leukemia with myelodysplasia related changes (AML-MRC) and elderly AML.
Methods
Totally 69 newly diagnosed AML-MRC and elderly AML treated with decitabine and priming regimen were enrolled. The effects of clinical characteristics, gene mutations and karyotype on remission rate and overall survival were investigated, as well as the effects of allogeneic hematopoietic stem cell transplantation on survival after remission.
Results
There were 39 and 10 cases achieving complete remission (CR) and partial remission (PR), respectively, with CR rate of 56.5% and overall response (OR) rate of 71%. Moreover, 15 cases had no response (NR), with NR rate of 21.7%. There were 5 cases of death within 4 weeks after treatment, with early mortality rate of 7.2%. The factors of peripheral white blood cell count, bone marrow blast proportion, doubling of platelets after treatment, and co-mutation of three or more myeloid genes, significantly affected CR and OR rates, while age significantly affected OR rate. TP53 mutation and platelets not doubling after treatment were independent prognostic factors affecting overall survival.
Conclusion
Combination of decitabine and priming regimen is effective in treating newly diagnosed AML-MRC and elderly AML.
Introduction
Acute myeloid leukemia (AML) is a type of heterogeneous hematopoietic malignant disease characterized by the clonal proliferation of myeloid blasts in peripheral blood, bone marrow and other tissues. AML with myelodysplasia related change (AML-MRC) and elderly AML have been considered to be two independent types that are difficult to treat. They are not sensitive to conventional chemotherapy and have poor prognosis, with median survival time of only about 1 year [Citation1, Citation2].
As a demethylation drug, decitabine has been currently approved for the treatment of AML patients who are not suitable for the intensive chemotherapy or having poor prognosis [Citation3]. Demethylation drugs at low doses could activate the silent tumor suppressor genes through demethylation to exert anti-tumor effects, while drugs at high doses may have cytotoxic effects [Citation4]. Priming regimen, firstly used in Japan, is effective and safe for the treatment of AML and myelodysplastic syndrome (MDS), particularly for high-risk and elderly patients [Citation5]. Although the combined effects of decitabine and priming regimen on AML-MRC or elderly AML have been reported, there are some controversies on their efficacy in AML-MRC or elderly AML [Citation6,Citation7].
In this retrospective study, the efficacy of low-dose decitabine combined with priming regimen in patients with newly diagnosed AML-MRC and elderly AML was investigated. The data of remission rate, survival and prognosis as well as their influencing factors were collected and analyzed. To the best of our knowledge, this is the first retrospective study investigating the application of decitabine in combination with priming regimen as the first-line therapy for AML-MRC and elderly AML. Our findings might provide evidence for the clinical treatment of AML-MRC and elderly AML.
Materials and methods
Study subjects
Totally 69 cases of patients with newly diagnosed AML-MRC and elderly AML admitted from February 2013 to February 2019 were enrolled in this study. They were diagnosed according to the International Working Group (IWG) criteria [Citation8]. The inclusion criteria were as follows: patients with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–3, the creatinine level and total bilirubin of ≤2 mg/dL. The exclusion criteria were: patients with acute promyelocytic leukemia; patients with other malignancy without remission; patients previously received chemotherapy (other than hydroxyurea) for any myeloid disorders. The bone marrow specimens were obtained from these patients. This study was performed in accordance with the Declaration of Helsinki. All patients signed written informed consent. This study was approved by the Institutional Review Board of our hospital.
Karyotype analysis
The bone marrow cells were cultured for 24–48 h, which were then collected and subjected to the R banding analysis. Abnormal karyotype was determined according to the International System for Human Cytogenetic Nomenclature (ISCN, 2013). If there is the same chromosome increase or structural abnormality in at least 2 cells, or the same chromosome loss in 3 cells, one abnormal clone was determined. Monosomal karyotype was defined as the presence of ≥2 autosomal monomers or 1 autosomal monomer combined with at least one structural abnormality in the same clone (except for loss of sex chromosomes). Complex karyotype was defined as the simultaneous presence of ≥3 unrelated chromosomal abnormalities.
Gene mutation detection
For the newly diagnosed AML patients treated after year 2016, 5 mL bone marrow fluid or peripheral blood was collected. Totally 34 reproducible candidate genes were selected and subjected to the second-generation targeted sequencing, including DNMT3A, ASXL1 TP53, TET2, and IDH1/2. DNA was extracted from bone marrow fluid or peripheral blood, which was then amplified using the Ampliseq multiplex PCR to construct the sample library. High-throughput sequencing was performed by Wuhan Kangshengda Medical Laboratory (China) on the Ion proton platform, and, after sequencing, the bioinformatics prediction was conducted according to the COSMIC database, to determine the mutation sites of pathogenic genes. The sequencing depth was 500×, with the sensitivity of 1%.
Induction therapy
For the induction therapy, the decitabine combined with a half-dose CAG regimen (D-CAG) was adopted. In detail, the therapy was given as follows: decitabine 15 mg m−2/d, intravenous drip, D 1–5; aclarubicin 10 mg/d, intravenous drip, D 4/6/8/10; Ara-C 30 mg, D 4–10; and G-CSF 5–10 ug/kg, subcutaneous injection, starting on D 3. The treatment course was determined based on the number of leukocytes/white blood cells (G-CSF should be stopped when white blood cells were >20 × 109/L). After treatment, supportive treatment was given according to the patient’s condition.
Treatment after complete remission
Patients who had achieved complete remission (CR) should continue to receive the original induction therapy regimen. These patients were subjected to the sequential administration of the standard ‘3 + 7’ regimen and medium-to-large-dose cytarabine regimen [0.5–2 g/(m2·times)] for a total of 4–6 cycles. A total of 12 patients underwent the allogeneic hematopoietic stem cell transplantation after CR.
Efficacy, adverse reaction and follow-up
At two weeks after treatment, the treatment efficacy was evaluated. The AML efficacy standards proposed by the International Working Group [Citation8] were used, including CR, partial remission (PR) and no response (NR), and overall response (OR = CR + PR). Early death referred to death within 4 weeks after starting the induction therapy. Overall survival (OS) was defined as the time from the date of disease diagnosis to death or the last follow-up, and disease free survival (DFS) was defined as the time from the day of CR to the first recurrence or death. Follow-up was conducted based on the inpatient medical records, outpatient medical records and/or with telephone interviews. The deadline for follow-up was March 31, 2020, with a median follow-up period of 32 months for the entire cohort.
Statistical analysis
The SPSS 20.0 statistical analysis software was used for statistical analysis. The chi-square test was used for analyzing categorical variables [n(%)], and the analysis of variance was used for the continuous variables [median (range)]. Kaplan-Meier method was used to evaluate survival and the difference in survival was analyzed with the Log-rank test. The COX proportional hazard model was used for the univariate and multivariate analysis. P < 0.05 was considered as statistically significant.
Results
Patient characteristics
The clinical characteristics of these 69 patients were retrospectively analyzed (). There were 28 cases of young AML-MRC, 21 cases of elderly AML-MRC, and 20 cases of elderly AML, with the median age of 62 years (ranging from 15 to 92 years). There were 35 males and 34 females. The median white blood cell count, hemoglobin, platelets, bone marrow blast proportions and risk categories according to karyotype are also shown in .
Table 1. Patient characteristics.
Overall efficacy and adverse reactions
A total of 69 AML patients received low-dose decitabine combined with priming regimen. Among them, 39 patients achieved CR, with the CR rate of 56.5%; 10 patients reported PR, with the OR rate of 71.0%; and 15 patients reported NR, accounting for 21.7% (). In terms of adverse reactions, except for 5 early deaths (accounting for 7.2%), the remaining patients had adverse events with varying degrees, including the bone marrow suppression and infection. However, they were all improved after active supportive treatment, and the overall adverse reactions were controllable ().
Table 2. Response and side effect during induction therapy.
Effects of clinical characteristics, gene mutations and karyotype on remission rates
The effects of clinical characteristics on remission rates were analyzed and are shown in . Our results showed that the number of white blood cells, the proportion of bone marrow blasts, and whether platelets double after treatment (without platelet transfusion for sucessive 7 days) affected both the CR rate and OR rates. However, age did not significantly affected the CR rate (P = 0.116), but it indeed had an impact on the OR rate. Next, the effects of gene mutations and karyotype were analyzed. Our results showed that remission rate for patients with co-mutation of three or more myeloid genes at the same time was significantly lower. However, other factors including the TP53 mutation, haplotype, and complex karyotype had no significant impact on the remission rate ().
Table 3. Effect of clinical characteristics on treatment response.
Table 4. Effect of cytogenetics and gene mutations on treatment response.
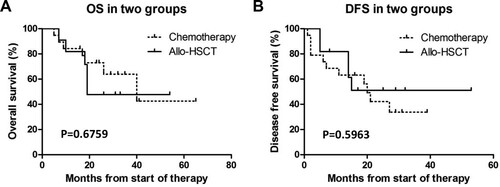
Effects of allogeneic hematopoietic stem cell transplantation on survival after complete remission
A total of 39 patients achieved CR, and 9 patients were lost to follow-up. Thus, a total of 30 patients had complete follow-up data. Among them, 11 received allogeneic hematopoietic stem cell transplantation, and the remaining 19 received chemotherapy as a consolidation treatment. Our results showed that the two consolidation treatment options had no significant effects on the OS and DFS of patients ().
Effects of clinical characteristics, gene mutations and karyotype on DFS and OS
Univariate and multivariate analysis was performed to analyze the factors affecting DFS and OS. Univariate analysis showed that age, white blood cell count, and doubling of platelets after treatment had significant impacts on DFS. However, the multivariate analysis showed that only doubling of platelets after treatment had a significant impact on DFS (). For the OS, univariate analysis showed that age, white blood cell count, bone marrow blast cell ratio, platelet doubling after treatment, TP53 mutation, and three or more myeloid tumor gene mutations at the same time affected OS. However, the multivariate analysis showed that only TP53 mutation and doubling of platelets after treatment had significant impacts on OS ().
Table 5. Cox regression analyses of risk factors for disease free survival (DFS).
Table 6. Cox regression analyses of risk factors for overall survival (OS).
Discussion
Newly diagnosed AML-MRC and elderly AML patients included in this study both had high-risk leukemia, and the remission time of conventional chemotherapy was short. Decitabine, as a demethylation drug, has become the first-line drug for the treatment of MDS. It also has been used for the treatment of AML. Cashen et al. [Citation9] treated 27 cases of newly-treated elderly AML with decitabine alone at a dose of 20 mg m−2/d for 5 consecutive days and found that the treatment efficiency was 26%, of which 7% of patients achieved CR. Compared with the low-dose cytarabine, decitabine did not increase the induction remission rate, and did not reduce the adverse reactions. Kantarjian et al. [Citation10] treated 485 newly diagnosed elderly AML patients with a single-drug regimen of decitabine for 5 days. They found that the CR rate was 17.8%, with no satisfactory effects. However, in recent years, it has been shown that the therapeutic effects of decitabine combined with priming regimen are better than those of single-agent decitabine or chemotherapy alone [Citation11,Citation12]. A meta-analysis of 24 randomized clinical trials showed that D-CAG in patients with moderate to high risk of MDS and AML had better clinical efficacy than single-agent decitabine and simple priming regimen [Citation11]. Li et al. [Citation12] found that the total response rate of 1 course of D-CAG regimen in treating newly diagnosed elderly AML patients was 82.4%, with the CR rate of 64.7%. Our findings herein are consistent with these results, showing that the combined priming regimens could improve the efficacy of decitabine in treating AML.
In this study, the factors affecting the remission rate of two high-risk types of AML treated with decitabine combined with priming regimen were investigated. In terms of clinical characteristics, the number of white blood cells, the proportion of bone marrow blasts, and doubling of platelets after treatment significantly affected the CR rate and OR rate. However, the age did not have a statistically significant effect on the CR rate, but had an impact on the OR rate. Age and high white blood cell count have always been the clinical factors for poor prognosis of AML. In line with these findings, Monica Bocchia et al. [Citation13] have reported the use of decitabine in the treatment of 306 elderly AML patients in the real world. They found that age and high white blood cell count were independent adverse prognostic factors. Bhavana Bhatnagar et al. [Citation14] used the 10-day regimen of decitabine to treat 45 patients with newly diagnosed AML who were not suitable for intensive chemotherapy. They showed that the only different pre-treatment parameter between effective and ineffective patients was the difference in the ratio of bone marrow blasts. Low-dose decitabine could promote the differentiation of megakaryocytes and the release of platelets, suggesting that the early platelet response may be an important predictor of clinical response in patients. In this study, patients with doubled platelets after treatment were more likely to have CR. This was similar to the clinical results by Van der Helm et al. [Citation15], showing that platelets doubling after azacitidine treatment could predict treatment response.
In terms of gene mutations and karyotype, our results herein showed that the remission rate of patients with co-mutation of three or more myeloid genes was significantly lower. The issue of co-mutation of myeloid tumor genes has not been clearly elucidated in major guidelines. However, the significance of co-mutation of myeloid tumor genes has gradually received more and more attention. A previous study has shown that the co-mutation pattern of FLT3, NPM1 and DNMT3A represented a group of patients with extremely poor prognosis [Citation16]. In this study, there were 2 cases of this co-mutation pattern. None of them achieved remission, and their survival time was extremely short. Qin et al. [Citation17] retrospectively analyzed the data from 201 AML patients, and found that as the increase of the number of gene mutations, the CR rate was decreased after the initial induction therapy. The above findings were all obtained based on the standard chemotherapy. For the first time in the study herein, similar findings were obtained based on the therapy of decitabine combined with priming regimen, suggesting that this therapy could not improve the prognosis of patients with this type of multi-gene co-mutation.
It has been shown that allogeneic hematopoietic stem cell transplantation can improve the poor prognosis of high-risk AML patients in the first CR [Citation18]. However, in this study, there were no significant differences in DFS or OS between the treatment with allogeneic hematopoietic stem cell transplantation and that with the decitabine combined with priming regimen in patients undergoing chemotherapy after achieving CR. The possible reasons are as follows: (1) This study was a single center and retrospective one with a small sample size and a short median follow-up period. The long-term efficacy of allogeneic transplantation needs further in-depth observation. (2) Previous studies focused on the comparison between the allogeneic hematopoietic stem cell transplantation and traditional chemotherapy. In this study, a comparison was conducted between transplantation and maintenance chemotherapy based on the decitabine combined with priming regimen. We speculate that this regimen could be used to improve the efficacy of treatment in these patients and prolong the patients’ survival, which were consistent with the results from a previous meta analysis [Citation11].
In this study, univariate analysis suggested that age, white blood cell count, and doubling of platelets after treatment had significant impacts on DFS, while multivariate analysis found that only doubling of platelets after treatment had a signficant impact on DFS. Univariate analysis of OS suggested that age, high white blood cell, high proportion of bone marrow blasts, platelets not doubling after treatment, TP53 mutation, co-mutation of three or more myeloid genes significantly affected the OS of patients. On the other hand, the multivariate analysis showed that only TP53 mutation and platelets not doubling after treatment were independent prognostic factors affecting the OS of patients. Although it has been reported that demethylating drugs such as decitabine could improve the remission rate of patients with TP53 mutation, they could not improve their OS [Citation19,Citation20], which is in line with our findings herein concerning survival of patients harboring TP53 mutation. Platelet doubling after treatment was a predictive indicator for patients to achieve remission. Multivariate analysis showed that it was an independent prognostic factor affecting DFS and OS of patients. Co-mutation of multiple genes is related to factors such as high white blood cell levels, advanced age, and high bone marrow blast cell ratio, so it did not show statistical significance in the multivariate analysis.
This study was a retrospective analysis with limited sample sizes. The conclusions herein need to be confirmed by further in-depth randomized controlled clinical trials.
In conclusion, our results showed that the combination of decitabine and priming regimen had a good overall effect on the newly diagnosed AML-MRC and elderly AML, with controllable adverse reactions. This program is more likely to achieve remission for AML patients who are young, with low white blood cell count, low bone marrow blast cell ratio, platelets doubling after treatment, and without co-mutation of three or more myeloid genes. Allogeneic hematopoietic stem cell transplantation after remission did not prolong the PFS and OS of patients with newly diagnosed AML-MRC and elderly AML. TP53 mutation and non-doubling of platelets after treatment were independent prognostic factors for AML-MRC and elderly AML.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Webster JA, Pratz KW. Acute myeloid leukemia in the elderly: therapeutic options and choice. Leuk Lymphoma. 2018;59:274–287.
- Koenig KL, Sahasrabudhe KD, Sigmund AM, et al. AML with myelodysplasia-related changes: development. Challenges, and Treatment Advances. Genes (Basel). 2020;11:845.
- Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:721–749.
- Contieri B, Duarte BKL, Lazarini M. Updates on DNA methylation modifiers in acute myeloid leukemia. Ann Hematol. 2020;99:693–701.
- Wei G, Ni W, Chiao JW, et al. A meta-analysis of CAG (cytarabine, aclarubicin, G-CSF) regimen for the treatment of 1029 patients with acute myeloid leukemia and myelodysplastic syndrome. J Hematol Oncol. 2011;4:46.
- Liu J, Jia JS, Gong LZ, et al. [Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in MDS-EB and AML-MRC]. Zhonghua Xue Ye Xue Za Zhi. 2018;39:734–738.
- Pepe S, Scalzulli E, Colafigli G, et al. Predictive factors for response and survival in elderly acute myeloid leukemia patients treated with hypomethylating agents: a real-life experience. Ann Hematol. 2020;99:2405–2416.
- Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649.
- Cashen AF, Schiller GJ, O'Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677.
- Zhang JL, Cao YP, Li JG. [Efficacy and safety of decitabine combined with CAG (cytarabine, aclarubicin, G-CSF) for patients with intermediate or high risk myelodysplastic syndrome and acute myeloid leukemia: a meta-analysis]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2019;27:494–503.
- Li J, Chen Y, Zhu Y, et al. Efficacy and safety of decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin in newly diagnosed elderly patients with acute myeloid leukemia. Oncotarget. 2015;6:6448–6458.
- Bocchia M, Candoni A, Borlenghi E, et al. Real-world experience with decitabine as a first-line treatment in 306 elderly acute myeloid leukaemia patients unfit for intensive chemotherapy. Hematol Oncol. 2019;37:447–455.
- Bhatnagar B, Duong VH, Gourdin TS, et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk Lymphoma. 2014;55:1533–1537.
- van der Helm LH, Alhan C, Wijermans PW, et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named patient programme. Br J Haematol. 2011;155:599–606.
- Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–2221.
- Qin W, Chao HY, Cai XH, et al. [Coexisting mutations in NPM1-mutated elderly adults with acute myeloid leukemia]. Zhonghua Yi Xue Za Zhi. 2019;99:3152–3157.
- Suciu S, Mandelli F, de Witte T, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003;102:1232–1240.
- Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375:2023–2036.
- Becker H, Pfeifer D, Ihorst G, et al. Monosomal karyotype and chromosome 17p loss or TP53 mutations in decitabine-treated patients with acute myeloid leukemia. Ann Hematol. 2020;99:1551–1560.