ABSTRACT
Objective
Our aim was to retrospectively assess the role of routine CT scans within the first year of follow-up with a limited surveillance policy prior to Lugano recommendations in diffuse large B-cell lymphomas (DLBCL) achieving complete metabolic remission (CMR). We also evaluated the type of relapse detection and exposure to CT scans within the first five years.
Methods
Patients diagnosed with DLBCL who achieved CMR after first-line immunochemotherapy were included. Imaging studies and medical records were thoroughly reviewed.
Results
Among 101 DLBCL patients in the first CMR, a total of 19 relapses were identified in the study period (18.8% of DLBCL patients included). Nine patients relapsed within the first year (47.4% of all relapses) but only 3 of them were detected by the 202 surveillance CT scans performed during this first year of follow-up.
Conclusions
Our real-world data provide clinically applicable results which are in agreement with the Lugano recommendations based on trial data, highlighting the lack of utility of routine CTs in DLBCL patients achieving CMR.
Introduction
Non-Hodgkin lymphomas (NHL) were the seventh leading cause of cancer incidence worldwide and the 11th cause of cancer deaths in 2015 [Citation1]. Among aggressive B-cell lymphomas, diffuse large B-cell lymphoma (DLBCL) is the two most common subtype [Citation2]. DLBCL accounts for approximately 24% of newly diagnosed NHL cases in the US [Citation3]. Clinical symptoms at presentation are highly variable but typically include rapidly enlarging lymphadenopathy and constitutional symptoms [Citation4–6]. Most patients with DLBCL require treatment at diagnosis and anthracycline-containing immunochemotherapy regimens are the standard of care [Citation7].
After the introduction in the late 60s of anthracycline-containing chemotherapy that achieved lymphoma complete remissions [Citation8], the practice of surveillance to detect relapse with imaging tests during follow-up became standard [Citation9]. Thus, performing periodic CT scans has been the standard practice for the follow-up of patients with lymphoma in the last 30 years. Recently, FDG-PET/CT scan has been introduced to assess end-of-treatment (EOT) response in lymphomas, and usefulness of the follow-up with CT scans of patients achieving complete metabolic response (CMR) has been questioned. Physical examination and blood tests, including serum lactate dehydrogenase (LDH), are recommended, along with clinical judgment [Citation10]. Routine CT scans during follow-up have been assessed in several observational studies, with the rationale that early detection of lymphoma relapse might have a favorable impact on survival [Citation11–15]. However, these retrospective studies have failed to show significant differences in survival derived from routine surveillance CT scans [Citation12–15]. A workshop, held in Lugano, that included major international lymphoma clinical groups discouraged the routine use of surveillance CT scans, reserving them only when clinically indicated [Citation10]. Nonetheless, the use of routine CT scans every 3 or 6 months during the first two after EOT and annually thereafter remains a common practice in many centers [Citation16].
In the last 15 years at our center, the follow-up surveillance for DLBCL was limited to exclusively two CT scans during the first year. Thereafter, no more routine CT scans were done unless clinical or laboratory suspicion of relapse. The main aim of this study was to assess the utility of a limited surveillance policy with routine CT scans in patients with DLBCL achieving CMR after immunochemotherapy.
Material and methods
We conducted a retrospective analysis of patients diagnosed with DLBCL between 2008 and 2018 included in the Hospital del Mar Lymphoma Registry. The study was carried out in agreement with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice [Citation17]. All included patients signed the informed consent, and privacy rights of human subjects were preserved. Approval by the local ethics committee was obtained.
Eligibility inclusion criteria were: DLBCL biopsy-proved diagnostic, treatment with curative intention and CMR assessed by PET/CT scan at EOT. Patients could be considered ‘lost to follow-up’ when no post-treatment visit after response assessment was found or who were lost to follow-up during the first year without evidence of relapse. These patients were not included in the analysis. Patients with transformed DLBCL from follicular lymphoma, chronic lymphocytic leukemia, marginal zone lymphoma or other DLBCL subtypes were included.
Baseline data at diagnosis such as physical examination, blood test findings including serum LDH levels, presence of bulky mass, Ann Arbor stage and International Prognostic Index (IPI) were reviewed.
Assessment of response by 18F-FDG PET: All PET scans were interpreted without any knowledge of the clinical data. In the first four years of the study (prior to Deauville criteria), all PET scans were scored either as positive or as negative. A positive result was defined as focal activity higher than that of the surrounding background tissue, with no similar activity seen on the contralateral side or increased activity in a location incompatible with normal anatomy. A negative result was defined as no pathologic 18F-FDG uptake at any site, including all sites of previously increased pathologic 18F-FDG uptake. Later, the Deauville criteria were incorporated in the evaluation of response in our Lymphoma Unit and, CMR was considered for scores 1–3 in the 5-point scale.
From 2008, the routine surveillance for aggressive lymphomas in our Lymphoma Unit included a restricted number of imaging tests. The surveillance guidelines were restricted to only two routine CT scans during the first year, performed at 3 and 12 months from EOT in patients with aggressive lymphomas who achieved CMR. No more scheduled CTs were planned in the follow-up of these patients. We reviewed the timing of CT scans performed within the first year of follow-up and, the cause of non-compliance was identified if applicable. We also calculated the number of CT scans performed within the first five years of follow-up and defined an accurate protocol adherence when two routine CT scans were performed within the first year. A ‘positive’ CT scan was considered when leading to a confirmatory biopsy, regardless of its result. Furthermore, we obtained a positivity rate of surveillance CT scans at each month (every three months within the first year), along with overall positivity rate within the first year. Positivity rate includes in the numerator routine CT scans considered as ‘positive’ following the previously explained criterion: all routine CT scans leading to a confirmatory biopsy, independently of relapse confirmation. Besides, diagnostic yield of surveillance CT scans within the first year was calculated as the ratio of biopsy-proved relapses identified solely by surveillance CT scan among the total number of CT scans performed during this time-lapse. Thus, the difference between positivity rate and diagnostic yield is the confirmation of relapse by a pathological biopsy, which is necessarily achieved to be part of the numerator of the diagnostic yield, while positivity rate includes in the numerator all surveillance CT scans which prompted the performance of a biopsy to rule out relapse.
Moreover, we categorized suspicion of relapse detection in the following groups: (1) abnormal physical examination; (2) blood test findings; (3) surveillance CT scan; (4) composite detection; (5) unscheduled CT scan; 6) other procedures (for instance, upper gastrointestinal endoscopy). The first finding was the one that assigned the category. The category named ‘composite detection’ was considered when suspicion of relapse was caused by more than one finding at the same time. Laboratory test findings include abnormalities in the following parameters: complete blood cells count, LDH, liver enzymes and peripheral blood smear. Other variables followed-up at relapse were like those evaluated at diagnosis.
Overall survival (OS) was measured from the date of treatment initiation to the date of death from any cause or the date of last visit. Progression-free survival (PFS) was calculated from the date of treatment initiation to the date of relapse, disease progression, death from any cause or the date of last visit. Cause of death was extracted from electronic medical records. Probabilities of OS, PFS and cumulative incidence were calculated using Kaplan–Meier method. Statistical analyses were performed using IBM SPSS Statistics 23.
Results
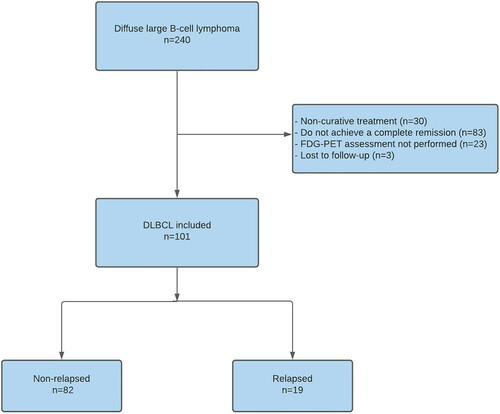
We identified 240 patients diagnosed with DLBCL between 2008 and 2018. One hundred one patients fulfilled all inclusion criteria and were enrolled in the study. The reasons for not being included in the study are shown in . Three patients who achieved CMR after treatment were transferred to other cities for personal reasons. These patients did not have any surveillance appointment and were considered as loss to follow-up.
Figure 1. Flowchart detailing causes of exclusion, number of patients included and number of relapses.
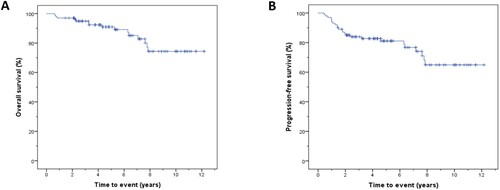
Patient characteristics at diagnosis and treatment are available in . Median follow-up was 59 months (IQR = 34–89.5 months). Mean OS and PFS were 125.3 months (95% confidence interval (CI) = 115.9–134.8 months) ((A)) and 112.9 months (95% CI = 101.6–124.2 months) ((B)), respectively.
Table 1. Patient characteristics at diagnosis and treatment.
A total of 19 relapses were identified, a figure that represents 18.8% of DLBCL patients included. Nine patients relapsed within the first year (47.4% of all relapses). The cumulative relapse incidence within the first year was 8.9%. According to type of relapse detection in the first year, 3 (33.3%) were diagnosed by CT scan, 4 (44.4%) by physical examination, 1 (11.1%) by upper gastrointestinal endoscopy and 1 (11.1%) by a combination of physical examination and blood test. Globally, 15.8% of relapses were diagnosed by institutionally recommended surveillance CT scans. Patient features at relapse are described in and further details about relapses are provided in .
Table 2. Patient characteristics at relapse.
Table 3. Type of suspicion of relapse within the first year and from the second year.
A total of 202 CTs were done in the first year, for an overall protocol adherence of 75.2%. The overall positivity rate in the first year was 2%, with only 4 positive CT scans in this time-lapse. At 3 months of EOT, 81 out of 101 patients had a CT scan. Only 1 out of the 81 CTs was considered positive, but a biopsy of the suspicious lesion did not confirm lymphoma relapse. At 12 months, 53 patients had a CT scan, for a protocol adherence of 47.5% and relapse was detected in only 1 case. Out of schedule, this means at 6 or 9 month, 68 CTs were done, but only in 2 cases was detected relapse. Diagnostic yield within the first year, defined as the ratio of biopsy-proven relapses identified exclusively by surveillance CT scan among the total number of CT scans performed, was 1.5% (95% CI = 0–3.2%; p < .001), since only three relapses were confirmed out of the total amount of CT scans performed. Additionally, as previously mentioned, a biopsy ruled out relapse in one patient with a positive routine CT scan at 3 months, leaving a false-positive rate of 25%. The median number of CT scans within five years of follow-up was 3 (range = 0–16). We identified 10 patients (9.9%) who were onto more than 8 CT scans during the first 5 year of follow-up. Overall exposure to CT scans is summarized in .
Table 4. CT scans exposure up to five years of follow-up.
Discussion
CT scans have remained common practice in the last three decades for the response evaluation and the follow-up of patients with lymphoma [Citation18]. This imaging technique was particularly relevant after the introduction of anthracycline-containing polychemotherapy in aggressive lymphomas because up to 50–60% of these patients could achieve long-term survival [Citation7,Citation9]. Surveillance policy typically included routine CT scans every 3–4 months during the first 2 years, and annually thereafter. During the last fifteen years, the surveillance protocol for lymphoma patients in CMR at our Lymphoma Unit included only 2 CT scans within the first year, performed at 3 and 12 months after EOT. This practice was mainly followed after introduction of response evaluation by PET-CT. The recent Lugano guidelines [Citation10] discouraged routine CT scans among asymptomatic patients achieving CMR, reserving them only when prompted by clinical features.
In our cohort of DLBCL patients, the cumulative relapse incidence within the first year was less than 10%, but in spite of the fact that about half of relapses occurred within the first year, the suspicion of progression was raised by CT in only a third of them. In fact, the diagnostic yield of surveillance CT scan within the first year was only 1.5%. Therefore, our real-world data in DLBCL patients are in accordance with the Lugano recommendations [Citation10], discouraging the use of routine CT scans for detecting a relapse in advance.
Beyond the impact of surveillance CT scans within the first year, we were also interested in analyzing the value of CT scans between the second and fifth year of follow-up. Fifteen relapses were identified from the second year, mostly by physical examination. With respect to CT scans, five relapses were detected by unscheduled CT scans and 1 by a combination of physical examination and CT scan. Then, we consider that surveillance by CT scan has also scarce benefit, if any, from the second year. In DLBCL patients, since most relapses occur within the first 2 years [Citation19], we would have required a considerable exposure to CT scans to detect just one relapse.
Another argument for surveillance CT scans might be the theoretically favorable impact on survival derived from early relapse detection, when disease is not widely spread. Nevertheless, since most DLBCL patients achieved a sustained CMR, our cohort did not provide us enough relapsed patients to perform a subgroup analysis from time of relapse. However, some observational studies have already addressed the impact of detection by surveillance CT scan on survival, obtaining no differences between symptomatic and asymptomatic patients detected by routine CT scan [Citation13–15]. Consequently, there are no data to clearly support routine imaging in asymptomatic DLBCL after first-line therapy, and especially in the PET-era. In follicular lymphoma in first CR, Goldman et al. have observed that most relapses were detected clinically [Citation18]. They did not find differences in OS according to the method of relapse detection (clinical vs radiologic detection through surveillance imaging) and therefore, they also suggest a limited role for routine surveillance imaging in asymptomatic follicular patients in first CR.
Another practice of limited evidence in detecting relapse is the performance of regular blood testing in the surveillance of DLBCL [Citation20]. Recently, Hawkes and cols have shown that blood tests do not reliably detect relapse in asymptomatic patients with aggressive lymphoma in CR [Citation21]. In our study, we only detected one patient in which relapse was suspected by abnormal blood test either with physical examination abnormality. Then, regular blood test should not be longer recommended in surveillance guidelines and, probably, this will improve the value of health care for patients.
Moreover, it is also remarkable that some negative effects have been described from this practice, especially the risk of secondary primary malignancies (SPM) due to exposure to radiation. In regard to this risk of SPM, two major contributors have been identified: radiotherapy (RT) and surveillance CT scans. The association between RT and the development of SPM is well-known for many years. This risk occurs not only in elderly patients but also in young adults and children. Of special interest are the findings of a phase III study in children with Hodgkin lymphoma where a significantly increased risk of SPM is observed in those who received RT [Citation22]. Conversely, the risk associated with performing multiple CT or other imaging tests is underestimated in the clinical practice. However, their association is unquestionable [Citation23]. Chien and cols have described the risk for SPM among NHL patients in relationship with exposure to CT scans, identifying that those patients who had more than 8 CT scans have double risk for developing SPM than those without [Citation24]. In our cohort, 8 patients were treated with RT and 11 had more than 8 CT scans within the first five years of follow-up, being at a higher risk for developing SPM. Interestingly, most patients having more than 8 CT scans were included in clinical trials. Because of this, we also believe that the number of imaging tests in clinical trials should be limited. For this, and in order to minimize the risk of radiation-induced SPM, other tools should be investigated for the follow-up of DLBCL patients. Liquid biopsy using next-generation-sequencing is a very promising technique to monitor treatment response of patients with DLBCL and its value in the follow-up of patients with DLBCL is under study by several groups. Preliminary results are very promising [Citation25,Citation26]. Concerning other potential negative effects related to surveillance by CT scan, Thompson and cols showed that this practice can be a source of anxiety and fear of recurrence, besides anxiety derived from disease itself [Citation27]. In our cohort, 1 false-positive CT scan led to a preventable biopsy at 3 months, being a possible source of anxiety. Nevertheless, our study was not designed to measure this effect. Additionally, a negative cost-effectiveness has been reported in association with common use of surveillance CT scans [Citation28].
This investigation accounts for several limitations. While the DLBCL cohort of patients achieving CMR was pretty large, given the low number of relapsed patients, we were unable to assess whether any subgroup of patients could specifically benefit from CT follow-up. However, our real-world data in a long-term follow-up cohort provide directly applicable results, which support the Lugano recommendations [Citation12] that were mainly based on clinical trial data.
In conclusion, in the PET–CT scan era, surveillance with CT scans is not useful among DLBCL patients in CMR after immunochemotherapy, reinforcing Lugano’s recommendations [Citation10] and supporting its application in the context of routine clinical practice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, regional, and National Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 Cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390.
- Teras LR, DeSantis CE, Cerhan JR, et al. US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;2016(66):443–459.
- Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94:604–616.
- Christopher AMLS M. HHS public access. Physiol Behav. 2016;176:100–106.
- Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer data base from 1998 to 2011. Am J Hematol. 2015;90:790–795.
- Grupo Español de Linfomas/Trasplante Autólogo de Médula Ósea. Guía de GELTAMO para Tratamiento del linfoma B difuso de célula grande (LBDCG). SEHH. 2016.
- Jones SE, Grozea PN, Metz EN, et al. Superiority of adriamycin-containing combination chemotherapy in the treatment of diffuse lymphoma. A southwest oncology group study. Cancer. 1979;43:417–425.
- Guppy AE, Tebbutt NC, Norman A, et al. The role of surveillance CT scans in patients with diffuse large B-cell non-Hodgkin’s lymphoma. Leuk Lymphoma. 2003;44:123–125.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068.
- Kallam A, Adusumalli J, Mph M, et al. Surveillance in patients with diffuse large B cell lymphoma. Mayo Clin Proc. 2020;95:157–163.
- Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32:3506–3512.
- Goldschmidt N, Or O, Klein M, et al. The role of routine imaging procedures in the detection of relapse of patients with Hodgkin lymphoma and aggressive non-Hodgkin lymphoma. Ann Hematol. 2011;90:165–171.
- Kang KW, Lee SR, Kim DS, et al. Lack of usefulness of computed tomography for surveillance in patients with aggressive non-Hodgkin lymphoma. PLoS One. 2018;13:1–14.
- El-Galaly TC, Jakobsen LH, Hutchings M, et al. Routine imaging for diffuse large B-cell lymphoma in first complete remission does not improve post-treatment survival: a danish–Swedish population-based study. J Clin Oncol. 2015;33:3993–3998.
- Tilly H, da Silva M G, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–v125.
- Dixon JR Jr. The international conference on harmonization good clinical practice guideline. Qual Assur. 1999;6:65–74.
- Goldman ML, Mao JJ, Strouse CS, et al. Surveillance imaging during first remission in follicular lymphoma does not impact overall survival. Cancer. 2021;127:3390–3402.
- Larouche JF, Berger F, Chassagne-Clément C, et al. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: clinical characteristics and outcome. J Clin Oncol. 2010;28:2094–2100.
- Piercey OA, Loh Z, Chan J, et al. Routine blood tests in asymptomatic patients with indolent lymphoma have limited ability to detect clinically significant disease progression. JCO Oncol Pract. 2020;16:e1315–e1323.
- Hawkes EA, Loh Z, Estacio O, et al. Routine blood investigations have limited utility in surveillance of aggressive lymphoma in asymptomatic patients in complete remission. Br J Cancer. 2018;119:546–550.
- Giulino-Roth L, Pei Q, Buxton A, et al. Subsequent malignant neoplasms among children with Hodgkin lymphoma: a report from the children’s oncology group. Blood. 2021;137:1449–1456.
- Brenner DJ, Hall EJ. Computed tomography – an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284.
- Chien SH, Liu CJ, Hu YW, et al. Frequency of surveillance computed tomography in non-Hodgkin lymphoma and the risk of secondary primary malignancies: a nationwide population-based study. Int J Cancer. 2015;137:658–665.
- Heimann P, Dewispelaere L. Indications of next-generation sequencing in non-hodgkin’s lymphoma. Curr Opin Oncol. 2020;32:391–397.
- Galimberti S, Genuardi E, Mazziotta F, et al. The minimal residual disease in non-Hodgkin’s lymphomas: from the laboratory to the clinical practice front. Oncol. 2019;9:528.
- Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in longterm lymphoma survivors. Ann Oncol. 2010;21:2262–2266.
- Huntington SF, Svoboda J, Doshi JA. Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol. 2015;33:1467–1474.