ABSTRACT
Objectives
To establish the experiences with and preferences towards existing thrombopoietin-receptor agonist (TPO-RA) treatments of individuals with immune thrombocytopenia (ITP) in the UK and Ireland, based on treatment attributes.
Methods
Responses from UK and Ireland individuals with ITP were collected in a pan-European online survey (TRAPeze, [Thrombopoietin-Receptor Agonist Patient experience survey]) from 18 September 2020 to 18 February 2021. TRAPeze was a survey of treatment preference regarding TPO-RAs (using a discrete choice experiment design), participant demographics, disease characteristics, treatment history, overall satisfaction with therapy, direct healthcare resource utilization and wider social impact.
Results
The survey was completed by 32 UK respondents. Characteristics with the greatest influence on preference towards TPO-RA treatments were method of administration (odds ratio (OR) 5.6, 95% confidence interval (CI) 3.2–10.1) and drug-food interactions (OR 3.2, 95% CI 1.8–5.7). Particularly, participants were more likely to select an oral tablet over a subcutaneous injection (OR 7.4, 95% CI 3.6–15.1) and a treatment without food restrictions rather than with food restrictions (OR 3.6, 95% CI 1.8–6.8).
Conclusion
This is the first study to quantify the preference of individuals with ITP towards TPO-RA treatment attributes and demonstrates preference for orally administered treatments, without drug-food interactions.
1. Introduction
Immune thrombocytopenia (ITP) is a rare autoimmune disorder characterized by a low platelet count, caused by antibody-mediated platelet destruction and impaired platelet production [Citation1]. ITP is diagnosed by a platelet count of <100 × 109/L [Citation2]. Individuals may present with signs of bleeding, such as purpura, petechiae, bruising and epistaxis [Citation3], with the risk and severity of bleeding events increasing in those who have a platelet count of <30 × 109/L [Citation4–6].
The current management strategy recommended by the American Society of Hematology (ASH) and the International Consensus Report (ICR) for ITP is to individualize treatment with the aim in adults to elevate platelet counts to >30 × 109/L in the majority [Citation4,Citation5]. By minimizing the risk of bleeding events, morbidity is reduced with an improvement in quality of life [Citation7,Citation8]. The initial therapy recommended for ITP are corticosteroids [Citation4,Citation5], however, prolonged treatment with corticosteroids is not recommended due to their potential adverse effects and high relapse rates [Citation6]. The recent international guidelines now recommend using the thrombopoietin-receptor agonists (TPO-RAs) as standard of care for those who relapse or are refractory to initial treatments for ITP [Citation4,Citation5]. However, during the COVID-19 pandemic, the use of TPO-RA as first-line therapy has been recommended for individuals who are negative for COVID-19 infection, due to concerns surrounding potential risks that corticosteroids pose in the development of COVID-19 infection or worsening of COVID-19 symptoms [Citation9]. There are currently three TPO-RAs licensed for ITP in Europe and the USA; romiplostim (Nplate®), eltrombopag (Revolade®) and avatrombopag (Doptelet®). Romiplostim is a subcutaneous injection, administered once weekly, either by a healthcare professional or self-administered at home [Citation10,Citation11]. Eltrombopag is an oral tablet taken once daily, two hours before or four hours after consuming any dairy products, antacids or mineral supplements [Citation12]. Avatrombopag is an oral tablet administered once a day with food, without any restrictions on meal composition [Citation13]. When this study began, only eltrombopag and romiplostim were available in the UK and Ireland.
The efficacy and safety of TPO-RAs are generally considered comparable, with several studies emphasizing that the major difference between these treatments lies in their product characteristics, such as method of administration and frequency of dosing [Citation14–16]. Whether TPO-RA choice may be driven by these differences has not been investigated to date. However, the choice could also be driven by prediction of response to baseline endogenous thrombopoietin (TPO) levels in individuals with ITP, with a greater likelihood of response to romiplostim than eltrombopag when TPO levels are slightly elevated above the normal range of 7–99pg/mL [Citation17]. Clinicians have suggested that TPO-RA treatment choice should be individualized and that, in addition to their own recommendations and medical experience, patient preferences can also play a role in treatment choice [Citation18], a strategy which is recommended by ASH and ICR guidelines [Citation4,Citation5]. There is limited evidence quantifying the differential impact of the product characteristics of TPO-RAs, such as method of administration, drug-food interactions, monitoring requirements and adverse events, on the preference of individuals with ITP. Therefore, the aim of the Thrombopoietin-Receptor Agonist Patient experience survey (TRAPeze) was to establish this through a discrete choice experiment and survey questions relating to participants’ experience with their TPO-RA treatments. To contextualize this, data regarding participant demographics, disease characteristics, treatment history, direct healthcare resource utilization and wider social impact were also captured. This paper describes the findings from the UK and Ireland cohort of the TRAPeze study.
2. Methods
2.1. Research design and survey recruitment to support Ethics Declarations during article submission
TRAPeze is an ongoing pan-European, cross-sectional, exploratory observational study of individuals with immune thrombocytopenia. The survey was available to participants in the UK and Ireland from 18 September 2020 to 18 February 2021.
Participants were recruited voluntarily by a patient advocacy group; the UK and Ireland ITP Support Association, via their social media platforms (Facebook, Twitter and Instagram) and mailing list.
Survey questions were based on advice from clinical experts and a targeted review of literature on TPO-RA product attributes, adverse reactions associated with TPO-RAs and ITP symptoms. The survey was presented as an online questionnaire on the web platform SurveyEngine®. Testing of the questionnaire was carried out prior to launch.
In accordance with the Medical Research Council/National Health Service (NHS) Health Research Authority, this study did not qualify as research and did not require formal NHS ethical approval. All participants were presented with a landing page detailnig the reason for the study, how participant data would be protected and stored, and assurance of anonymity, which they had to agree to in order to take part in the survey.
The TRAPeze survey consisted of two sections: a section on treatment preference (using discrete choice experiment design) and a section on disease burden. The discrete choice experiment was designed to elicit participant preference towards TPO-RA product characteristics and consisted of 10 sets of scenarios. For each set, participants were asked to choose between two hypothetical treatment scenarios. Each hypothetical treatment scenario was depicted by a list of TPO-RA product attributes, that alternated between sets (Table S1).
The remainder of the survey; the disease burden section, consisted of 101 questions regarding respondent demographics, disease characteristics, treatment history, overall satisfaction with therapy and impact of ITP on work and productivity, personal life and healthcare resource utilization.
Responses were available as radio buttons (single choice only), checkboxes (multiple choice possible), dropdown lists (single choice only) or free text. Some questions featured skip logic and almost all questions were optional, with participants able to skip or respond with ‘prefer not to say’. It was compulsory for participants to answer the treatment preference section and questions regarding which TPO-RA participants had currently or previously received.
All respondents met the following inclusion criteria; aged 18 or over, formally diagnosed with primary ITP according to ASH and ICR guidelines [Citation4,Citation5], currently receiving or previously received a TPO-RA for a minimum of 3 months, with at least some of the treatment received in the last 12 months. At the time that this study was conducted, only two TPO-RAs; romiplostim and eltrombopag, were available for individuals with ITP in the UK and Ireland, therefore these were the only TPO-RAs included in the survey questions.
2.2. Data processing
Responses were retrieved in Excel format from SurveyEngine®. Analysis of the treatment preference section was performed for all survey responses which included an answer to the question regarding which TPO-RA respondents are currently or had most recently received (question number 34 in the disease burden section). However, descriptive analysis of the disease burden section was only performed for responses which included an answer to how disease progression since diagnosis has changed the impact of ITP on relationships with partners, family members and friends (question number 100 in the disease burden section).
2.3. Data analysis
The responses from the treatment preference section were analysed using a mixed logit model to estimate the odds of participant preference towards TPO-RA product characteristics, with the OR and 95% CI reported in this paper.
Descriptive analysis of the responses to the disease burden questions is reported as total counts, frequency of responses and summary statistics (mean and standard deviation [SD], or median) of this cohort.
3. Results
3.1. Demographics
There were 32 respondents in total, of whom all completed the treatment preference section and 31 completed the section on disease burden, i.e. they completed up to question number 100 in the disease burden section. Of the UK and Ireland cohort (female 65% and male 35%), the majority of respondents (81%, n = 25) were aged over 50 at the time of survey completion, with a mean (SD) age of 57.6 (14.8) years. The average age of respondents at the time of ITP diagnosis was 48.7 (16.9) years and the mean time since diagnosis was 8.9 (11.2) years. There were 29 (95%) participants with chronic ITP and 2 (6%) participants with persistent ITP. All participants were white (n = 31) and almost all were British (n = 29), with two participants being Irish.
3.2. Disease characteristics
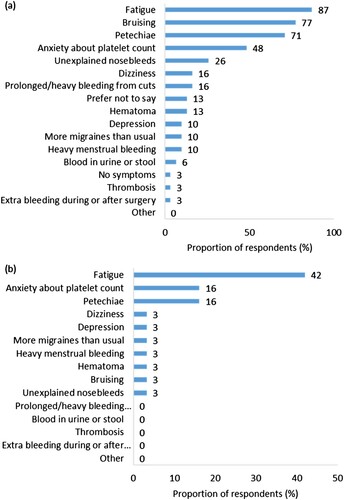
Participants reported experiencing a mean (SD) of 4 (1.8) symptoms when affected by ITP. The five most commonly reported symptoms of ITP were fatigue (87%, n = 27), bruising (77%, n = 24), petechiae (71%, n = 22), anxiety about their platelet count (48%, n = 15) and spontaneous nosebleeds (26%, n = 8) ((a)). The symptom ranked as having the most negative impact on quality of life was fatigue (42%, n = 13), followed jointly by anxiety about platelet count (16%, n = 5) and petechiae (16%, n = 5) ((b)).
Figure 1. (a) Signs and symptoms of ITP experienced by respondents (n = 31). (b) Signs and symptoms of ITP ranked most negatively impactful on quality of life by respondents (n = 30, one respondent reported experiencing no symptoms).
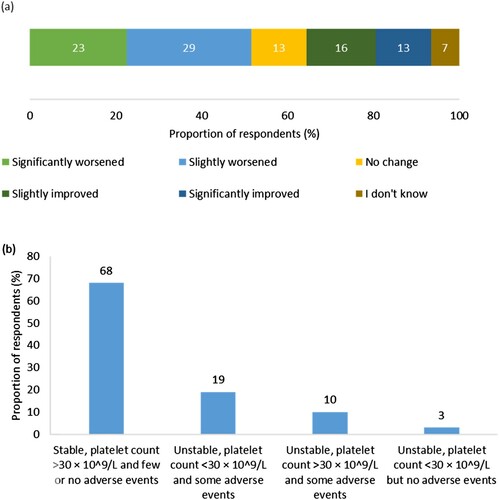
When asked to score their current health state on a scale of 1 (very poor) to 10 (excellent), the mean score participants gave was 7.3 (2.3). However, over half of the participants (52%, n = 16) reported that their condition had slightly or significantly worsened since their diagnosis ((a)). Over two-thirds of the participants (68%, n = 21) reported that their condition had been stable, with few or no adverse events, in the 3 months prior to survey completion, whilst 32% (n = 10) reported that their condition was unstable and associated with low platelet count, adverse events or both ((b)).
Figure 2. (a) Respondents’ perception of change in ITP condition since diagnosis (n = 31). Percentages may not add up to 100 due to rounding. (b) Respondents’ perception of stability of ITP condition in the 3 months prior to survey completion (n = 31).
All respondents (n = 31) were aware of their last recorded platelet count, the mean of which was 93.8 (59.0) × 109/L. Twenty-six per cent (n = 8) of respondents stated that their platelet count was <50 × 109/L, of which three reported a platelet count of <30 × 109/L.
3.3. Treatment patterns
About half of respondents (52%, n = 16) stated that their current or most recent TPO-RA treatment was romiplostim, whilst 48% (n = 15) stated this to be eltrombopag ((a)). The mean (SD) duration respondents had been receiving their current or most recent TPO-RA treatment was 2.4 (1.8) years ((b)). Only one participant had stopped taking their most recent TPO-RA, due to loss of response to treatment.
Figure 3. (a) Proportion of respondents (%) currently or most recently receiving romiplostim or eltrombopag (n = 31). (b) Duration (years) that respondents have been treated with their current or most recent TPO-RA (n = 31). The endpoints of the whiskers = minimum and maximum values; the points where the whiskers meet the box = first and third quartiles; the line in the box = median; the cross in the box = mean; the dot = outlier. (c) Proportion of respondents (%) who have previously received romiplostim or eltrombopag (n = 13). (d) Reasons respondents discontinued their previous TPO-RA treatment (n = 13). Percentages may not add up to 100 due to rounding.
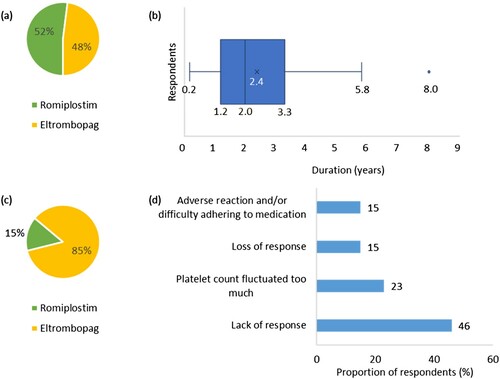
There were 13 participants who had switched from another TPO-RA, of which 85% (n = 11) had switched from eltrombopag ((c)). The most common reason for discontinuing their previous TPO-RA treatment was due to a lack of response to treatment (46%, n = 6) ((d)).
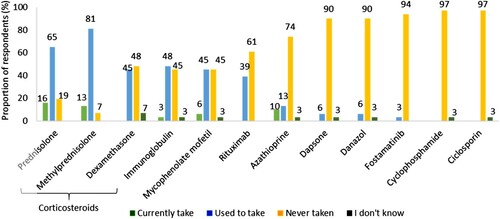
Participants (n = 31) reported that they were currently receiving between 1 and 4 therapies for ITP, with a median of 1. Other than TPO-RAs, corticosteroids were the most commonly prescribed treatment with 30 participants having reported that they were currently or had previously received a corticosteroid, most frequently prednisolone (81%, n = 25). Other commonly prescribed treatments for ITP included immunoglobulin (52%, n = 16) and rituximab (39%, n = 12) (). Only one participant had undergone a splenectomy since their ITP diagnosis. Changes were made to the treatments of three participants to prevent immune suppression as a result of the COVID-19 pandemic.
Figure 4. Other therapies that respondents currently take, used to or have never taken for ITP (n = 31). Percentages may not add up to 100 due to rounding.
The majority of respondents had received their blood tests at a hospital (67%, n = 22), with 32% (n = 10) receiving blood tests weekly. Few respondents had hospital admittances (26%, n = 8), visited the general practitioner (16%, n = 5) or utilized the mental health services (7%, n = 2) as a result of ITP in the 12 months prior to survey completion. However, all respondents (n = 31) had visited a specialist consultant for ITP, with over a third (32%, n = 10) making > 10 visits in the 12 months prior to survey completion. The distance travelled one-way to attend these specialist consultant appointments was less than 30 miles for all respondents (n = 31).
3.4. TPO-RA treatment experiences and preferences
In the disease burden section of the survey, participants were asked to what degree they agreed with statements relating to their current or most recent TPO-RA treatment to ascertain their satisfaction with their treatment. Over two-thirds of the participants agreed or strongly agreed that their current or most recent TPO-RA treatment was effective in maintaining their platelet production (68%, n = 21), preventing bleeding events (74%, n = 23) and treating their ITP symptoms (71%, n = 22). However, only 19% (n = 6) felt that their treatment increased their energy levels. Almost half of participants (48%, n = 15) agreed or strongly agreed that changes to their daily routine had to be made to take their TPO-RA, whilst 74% (n = 23 [Romiplostim, n = 14; Eltrombopag, n = 9]) disagreed or strongly disagreed that they had made changes to their diet for their treatment ().
Figure 5. Respondents’ degree of agreement to statements regarding their current or most recent TPO-RA treatment (n = 31). Percentages may not add up to 100 due to rounding.
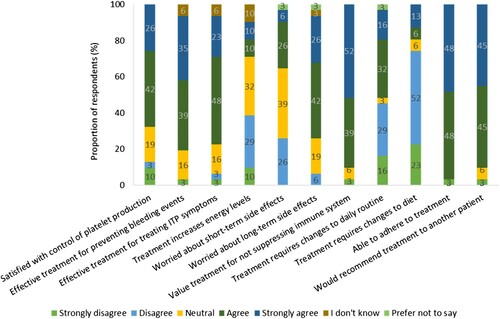
Most respondents (87%, n = 27) were satisfied with the mode of administration of their current or most recent TPO-RA treatment. Key reasons provided for TPO-RA treatment type satisfaction were lack of dietary restrictions in 13% (n = 4), preference for oral administration over subcutaneous injections in 19% (n = 6), and ease of administration in 23% (n = 7) of patients.
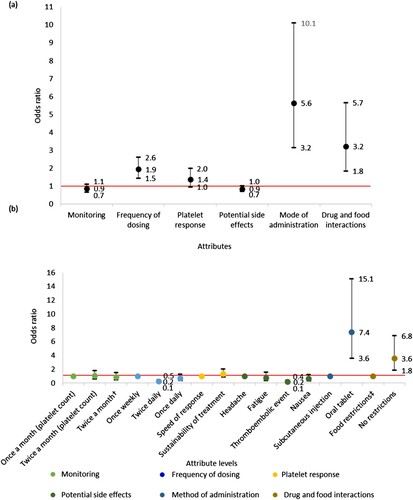
Results from the treatment preference section indicated that the product characteristics which were most likely to influence TPO-RA preference were method of administration (OR 5.6, 95% CI 3.2–10.1) and drug-food interactions (OR 3.2, 95% CI 1.8–5.7) ((a)). When comparing between attribute levels, it was found that participants were 7.4 (95% CI 3.6–15.1) times more likely to select an orally administered treatment over a subcutaneous injection. The odds of participants selecting a treatment without over a treatment with food restrictions was 3.6 (95% CI 1.8–6.8) ((b)).
Figure 6. (a) Association between TPO-RA attributes and participant preference towards TPO-RA treatments (n = 31). The red line indicates no effect (odds ratio = 1). The black lines indicate the lower to upper confidence intervals. (b) Association between TPO-RA attribute levels and participant preference towards TPO-RA treatments (n = 31). The red line indicates no effect (odds ratio = 1). For each attribute category, the first attribute level plotted is the reference level. Upper CI, upper confidence interval; the black lines indicate the lower to upper confidence intervals. †Two separate tests: one for measuring platelet count and one for liver function. ‡Treatment must be taken 2 h before or 4 h after food containing dairy products or calcium; indigestion remedies (antacids); or mineral supplements.
Discussion
Participants displayed a high understanding of their condition, with all participants aware of their last recorded platelet count, as well as their treatment history. They were also able to provide responses regarding changes to their treatment patterns as a result of the COVID-19 pandemic. This suggests that future clinical data may be gathered in a similar manner to this study.
Many respondents ranked fatigue as the most negatively impactful symptom of ITP on quality of life, which is consistent with findings from wider literature [Citation19,Citation20], and few agreed that the TPO-RA treatments they were receiving or had received resulted in an increase in their energy levels. However, these TPO-RAs were mostly considered to be effectively maintaining platelet counts and treating respondents’ ITP symptoms. This was supported by the fact that less than a third of respondents possessed a platelet count of <50 × 109/L, over two-thirds felt that their condition had been stable in the 3 months prior to survey completion and their current health state was also perceived to be fairly good, with scores averaging above 7 out of 10. The effectiveness of TPO-RAs in maintaining platelet production can be beneficial for those who experience anxiety about their platelet count, particularly as this was ranked as the second most impactful symptom of ITP. The majority of the respondents in this cohort have chronic ITP and have lived with this disease for almost 9 years, with more than half reporting that their condition had slightly or significantly worsened since diagnosis. As individuals with ITP experience a wide range of symptoms, with varying degrees of impact, a treatment which is effective in preventing these symptoms can greatly improve their quality of life.
Some participants visited specialist consultants more than 10 times in a year and received blood tests weekly, indicating that ITP is a long-term disease that consistently requires management. This is emphasized by the discontinuation of previous TPO-RA treatments due to inadequate response (either because participants became refractory to or never responded to treatment), as well as previous and concurrent prescriptions of other ITP treatments. Furthermore, participants had also received immunoglobulin and rituximab, both of which are recommended emergency treatments when an increase in platelet count is urgently required or in the case of life-threatening bleeding [Citation5], thus indicating the ongoing risks faced by individuals with ITP.
This study found that method of administration and drug-food interactions are strong drivers of TPO-RA choice in individuals with ITP in the UK and Ireland. This was shown in a discrete choice experiment (treatment preference section) and supported by responses (disease burden section) showing that satisfaction with eltrombopag was due to it being orally administered, rather than injected, and satisfaction with romiplostim was due to the absence of dietary restrictions.
Method of administration appeared to be a stronger driver of choice than drug-food interactions for three reasons. First, in the discrete choice experiment, participants were around 7 times more likely to choose an oral tablet over a subcutaneous injection but 4 times more likely to choose a treatment without food restrictions than with.
A second reason why method of administration appeared to be a stronger driver of choice than drug-food interactions is because eltrombopag was most frequently initiated first in respondents who had switched TPO-RAs. If, as studies have suggested [Citation18], prescription of TPO-RAs are influenced by patient preference, individuals may prefer to take eltrombopag first as it is orally administered. Therefore, although drug-food interactions are a driver of choice, they may not be as significant as method of administration.
The final reason that method of administration seems to be a stronger driver of choice than drug-food interactions is that 9 of the 23 individuals who did not feel that changes to diet were made for their treatment were currently receiving or had most recently received eltrombopag. This may be a result of participants administering their treatment before going to bed at night, as can be recommended [Citation6,Citation21]. Thus, the potential for drug-food interactions associated with eltrombopag may not be as considerable a concern as method of administration.
Although studies that investigate the perspective of individuals with ITP on disease burden, quality of life and general perspectives towards treatments have been conducted [Citation22,Citation23], this is the first study which explores their preferences towards TPO-RA treatments.
Limitations
ITP is a rare disease, with only 4116 individuals with ITP entered into the UK registry between 2007 and 2020 [Citation24]. Of these individuals, only 9.66% received eltrombopag and 5.99% received romiplostim during this period [Citation24]. As participants were required to have received a TPO-RA for at least 3 months in the 12 months prior to participation, the resulting sample size for the UK and Ireland was small, but it is believed that the proportion that has been captured may be considered representative. Furthermore, at the time that this study was conducted, avatrombopag was not licensed for treating ITP in the UK and Ireland. Therefore, treatment experiences and preferences for all available TPO-RAs have not been captured. This limits the representativeness of the cohort and prevents more granular analyses. However, this cohort is part of an ongoing pan-European study, which will allow for wider population participation and more in-depth analyses in the future.
This study was conducted virtually, thus making it difficult to verify that participants met the eligibility criteria. Participation of individuals who were not computer literate may have been limited; however, family members, carers and friends were allowed to assist participants in the completion of the survey. Given the challenges that arose from the COVID-19 pandemic, this may have been the most suitable approach to conduct the study.
Conclusion
This is the first study that quantifies the preference of individuals with ITP towards TPO-RA treatment attributes, with an aim to facilitate improved patient-centred ITP management. The findings from the UK and Ireland cohort of the TRAPeze study indicate that individuals with ITP consider method of administration and drug-food interactions important characteristics when choosing a TPO-RA treatment which suits them. Particularly, participants appeared to prefer a TPO-RA treatment which is void of dietary restrictions and orally administered; characteristics which are individual to romiplostim and eltrombopag, respectively, but combined in treatments such as avatrombopag that are now licensed for treating ITP in the UK and Ireland. Switching between TPO-RA agents is common practice, but further research into the reasons for switching will also be important for clinicians prescribing TPO-RAs in the future. The responses also indicate that ITP is a long-term burdensome disease that impacts the quality of life, with the symptom of fatigue persisting, regardless of treatment. Thus, it is essential that TPO-RA treatments chosen for individuals with ITP not only effectively maintain platelet counts and help to alleviate symptoms, but also impacts their quality of life in the smallest way possible. To gain further understanding of the potential drivers of treatment outcomes and quality of life for individuals with ITP, the TRAPeze study is currently being undertaken in multiple European countries.
Data accessibility statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental Material
Download MS Word (23.3 KB)Acknowledgements
The authors would like to thank all the participants who contributed to this survey and the patient advocacy group UK and Ireland ITP Support Association for their help in recruiting participants.
Disclosure statement
VM reports consultancy fees from Amgen, Bayer, Novartis and Sobi and research funding from Grifols. AN reports honoraria from Amgen, Angle, Argenx, Dova, Novartis, Ono and Shionogi, consultancy fees from Amgen, Angle, Argenx, Dova, Grifols, Novartis and Shionogi and research funding from Amgen, Novartis and Rigel. MM reports consultancy fees from Novartis, Sobi and UCB.KW and JN are full-time employees of Sobi. PM, EG and TW are employed by Wickenstones Ltd, a company that received consultancy fees from Sobi.
Additional information
Funding
References
- Li J, Ma S, Shao L, et al. Inflammation-related gene polymorphisms associated with primary immune thrombocytopenia. Front Immunol. 2017;8:744.
- Khan AM, Mydra H, Nevarez A. Clinical practice updates in the management of immune thrombocytopenia. Pharm Ther. 2017;42(12):756–763.
- Kayal L, Jayachandran S, Singh K. Idiopathic thrombocytopenic purpura. Contemp Clin Dent. 2014;5(3):410–414.
- Neunert C, Terrell DR, Arnold DM, et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866.
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817.
- Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia – current diagnostics and therapy: recommendations of a joint working group of DGHO, OGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(Suppl. 5):1–30.
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393.
- Arnold DM. Bleeding complications in immune thrombocytopenia. Hematol Am Soc Hematol Educ Program. 2015;2015:237–242.
- Pavord S, Thachil J, Hunt BJ, et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Br J Haematol. 2020;189(6):1038–1043.
- Schipperus M, Kaiafa G, Taylor L, et al. Assessment of self-administration of romiplostim in patients with immune thrombocytopenic purpura after receipt of home administration training materials: a cross-sectional study. Drug Saf. 2019;42(1):77–83.
- EMC. Romiplostim SmPC 2020. Available from: https://www.medicines.org.uk/emc/product/567/smpc.
- EMC. Eltrombopag SmPC 2021. Available from: https://www.medicines.org.uk/emc/product/508/smpc.
- EMC. Avatrombopag SmPC. 2021.
- Cooper N. State of the art – how I manage immune thrombocytopenia. Br J Haematol. 2017;177(1):39–54.
- Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol. 2019;10. doi:https://doi.org/10.1177/2040620719841735.
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6):1112–1123.
- Al-Samkari H, Kuter DJ. Thrombopoietin level predicts response to treatment with eltrombopag and romiplostim in immune thrombocytopenia. Am J Hematol. 2018;93(12):1501–1508.
- Thachil J, Bagot C, Bradbury C, et al. A United Kingdom immune thrombocytopenia (ITP) forum review of practice: thrombopoietin receptor agonists. Br J Haematol. 2018;180(4):591–594.
- Diraimo J, Kruse C, Lambert MP, et al. Impact of therapy choice on fatigue in adults with immune thrombocytopenia. Blood. 2020;136(Supplement 1):19–20.
- Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol. 2021;96(2):188–198.
- HPRA. Your practical guide to RevoladeTM (eltrombopag). 2016.
- Kruse C, Kruse A, Watson S, et al. Patients with immune thrombocytopenia (ITP) frequently experience severe fatigue but is it under-recognized by physicians: results from the ITP World Impact Survey (I-WISh). Blood. 2018;132(Supplement 1):2273.
- Cooper N, Kruse C, Kruse A, et al. Patient perceptions on splenectomy outcomes: results from the ITP World Impact Survey (I-WISH): PF714. HemaSphere. 2019;3:312.
- Raheja P, Miah H, Miah A, et al. Therapeutic landscape in primary immune thrombocytopenia over the last three decades in the United Kingdom (UK adult primary ITP registry project) (PB0818). Res Pract Thromb Haemost. 2021;5(Suppl 1).
