ABSTRACT
Introduction
Cytokine release syndrome (CRS) is a common complication after T-replete HLA haploidentical hematopoietic cell transplantation (haplo-HCT) with PTCy. We aim to assess the incidence, severity, and impact of CRS on clinical outcomes of patients who received haplo-HCT using Beijing Protocol.
Methods
This was a single-enter retrospective analysis of 286 subjects who received haplo-HCT with Antithymocyte Globulin (ATG).
Results
We identified 147/268 (54.9%) patients who developed CRS, grade 1 CRS (32.5%) and grade ≥2 CRS (22.4%). Eight patients developed severe CRS. The incidence and severity of CRS did not show significant discrimination among patients who received different doses of ATG. By multivariable analysis, age and the disease status at transplantation were significantly associated with the occurrence of CRS (p =.000 and p = .021). In the univariate analysis for the severity of CRS, compared with CRS grade ≥2, patients with CRS grade 0-1 had higher 1-year overall survival (OS) (p = .009). The cumulative incidence of 100-day grades II-IV acute GVHD was 12.4%. The incidence did not show significant differences between patients with CRS or not. The devolvement of CRS is associated with worse OS, inferior disease-free survival, and higher nonrelapse mortality significantly. But the result appeared to be limited to patients in uncomplete remission status before transplantation.
Discussion and Conclusions
CRS is less frequent and milder with a protocol based on ATG. CRS can potentially affect the outcomes after haplo-HCT especially for patients in an uncomplete remission. Prospective clinical trials are needed to provide an appropriate scheme for CRS prophylaxis.
Introduction
Haploidentical hematopoietic cell transplantation (haplo-HCT) is increasingly used for the treatment of patients with hematologic malignancies who lack a suitable HLA-matched donor[Citation1,Citation2]. In the past, high transplant-related mortality due to severe graft-versus-host disease (GVHD) and delayed immune reconstitution had restricted the use of haplo-HCT[Citation3,Citation4]. However, outcomes have significantly improved with the advent of approaches such as the Beijing Protocol using a combination of granulocyte colony-stimulating factor (G-CSF) mobilized bone marrow or peripheral blood stem cells, as well as antithymocyte globulin (ATG) administration for the prophylaxis of GVHD and graft rejection[Citation5,Citation6]. Survival rates among patients who undergo haplo-HCT including ATG-based regimens are comparable to those following HLA-matched sibling transplantation or unrelated donor transplantation[Citation7].
Fever in the first several days after haplo-HCT is a frequent occurrence[Citation8–11]. This febrile reaction is believed to be a cytokine-mediated phenomenon released by macrophages, which has now been defined as the cytokine release syndrome (CRS). CRS is well-recognized toxicity associated with T cell–engaging therapies such as chimeric antigen receptor–modified (CAR) T cell[Citation12–14]. The CTCAE v5.0 defines CRS as "a disorder characterized by fever, tachypnea, headache, tachycardia, hypotension, rash, and/or hypoxia caused by the release of cytokines". Post haplo-HCT CRS is believed to be secondary to a rapid proliferation of alloreactive T cells and the associated elevated circulating levels of several cytokines, including IL-6, TNF-α, and IFN-γ12. Imus et al.[Citation15] reported a large retrospective experience on CRS after haplo-HCT using peripheral blood (PB) allografts and post-transplant cyclophosphamide (PTCy) for GVHD prophylaxis. The recorded incidence of CRS and severe CRS was up to 90% and 17%. Half of the patients with severe CRS died in the first 6-months after transplantation. Abboud et al.[Citation16] investigated the incidence of CRS in recipients who received haplo-HCT and reported that 87% of patients met the criteria for CRS, 12% of which was grades 3–4 accompanied by high associated mortality. However, Salas et al.[Citation17] discovered that dual T cell depletion with ATG and PTCy for GVHD prophylaxis in haplo-HCT with PB allografts was associated with a much lower incidence of CRS and an absence of severe CRS. CRS occurred in 31.3% of recipients (grades 1 or 2).
Multiple clinical series have reported an increased incidence of high-grade fevers early after haplo-HCT. However, haplo-HCT platforms in these articles was based on PTCy. Consequently, we performed a retrospective study to assess the incidence, severity, and impact of CRS on clinical outcomes in haplo-HCT patients using Beijing Protocol with ATG.
Methods
Patients
Clinical data of 287 consecutive patients who underwent ATG-based T cell- replete, related-donor haplo-HCT between March 2013 and December 2018 at the Union Hospital of Huazhong University of Science and Technology were retrospectively analyzed. This study was conducted after Institutional Review Board approval. The World Health Organization’s classification of hematologic malignancies was applied [Citation18]. Patients who had undergone previous allogeneic transplantation were excluded from consideration. Patients with the microbiologically confirmed infection before day +14 were excluded from the analysis.
GVHD prophylaxis and conditioning regimens
All patients received uniform GVHD prophylaxis with rATG, tacrolimus, short-term methotrexate, mycophenolate mofetil, and basiliximab. Tacrolimus was administered orally twice daily at a dose of 0.05 mg/kg/d from day −3 to maintain blood levels between 5 and 10 ng/mL until day +60. If no aGVHD occurred, tacrolimus was tapered by 5% weekly and discontinued on the day +180. MTX was administered intravenously at dosages of 15 mg/m2 on day +1 and 10 mg/m2 on day +3, +6 and +11. Mycophenolate mofetil (1000 mg/d, orally twice daily) was administered from day +7. MMF was discontinued around day +60 if the patient was engrafted and free of GVHD, aGVHD and cGVHD were graded according to the consensus criteria [Citation19,Citation20]. Anti-CD25 MoAb (Basiliximab) was given intravenously at a dose of 20 mg on day 0 and days +4 [Citation21,Citation22]. ATG (Thymoglobulin, Sanofi, Paris, France) were administered intravenously at a dose of 2, 2.5 mg/kg, 3 mg/kg daily from day −3 to d −1. Conditioning regimens classified based on consensus criteria were mainly myeloablative conditioning [Citation23].
Antimicrobial prophylaxis and surveillance
Antimicrobial prophylaxis consisted of ganciclovir 5 mg/ kg twice daily from day −10 to −2, moxifloxacin 400 mg per day, and oral fluconazole from day −10 until engraftment. Patients received cotrimoxazole twice daily from the time of neutrophil recovery to 6 months [Citation22]. Monitoring of cytomegalovirus (CMV) DNA by qPCR in blood was performed weekly beginning with neutrophil recovery and continuing until day +100 [Citation21].
Definitions and study endpoints
CRS was defined and graded using the criteria described by Lee et al. [Citation24]. Symptoms occurring before day +14 were included. The diagnosis of CRS must preclude infections by utilizing appropriate clinical, radiographic, and/or laboratory investigations. In addition, CRS was distinguished from engraftment syndrome, which can also present as noninfectious fever and capillary leak, by onset before day +14 and the absence of skin rash [Citation25]. Disease Risk Index (DRI) [Citation26] was calculated based on established definitions. Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) of above 0.5 × 10^9/L. Platelet engraftment was defined as the first of 7 consecutive days with a count of above 20 × 10^9/L without platelet transfusion.
All infections were confirmed microbiologically. Bacterial infections were identified with positive blood culture. CMV infection was defined as any level of detectable CMV DNA by PCR. CMV reactivation, recurrence, and disease were diagnosed according to the criteria proposed by Ljungman et al. [Citation27]. Invasive fungal infections were confirmed by the classification proposed by the Infectious Disease Society of America 2016 update [Citation28].
Overall survival (OS) was defined as the time from transplantation until death from any cause, and surviving patients were censored at the last follow-up. Relapse was defined as disease recurrence or progression. Nonrelapse mortality (NRM) was defined as death without prior evidence of disease relapse. NRM was considered a competing event for calculating the cumulative incidence of relapse. Disease-free survival (DFS) was defined as the time from transplantation to either disease relapse or death. Patients alive and relapse-free were censored at the last follow-up.
Statistical analysis
Patient demographics and clinical characteristics were summarized using frequencies with percentages for categorical variables and median with range for continuous outcomes and compared between CRS severity (Grade 0 vs Grade1 vs Grade 2-3) cohorts using the Kruskal–Wallis test. Survival distributions were estimated using the Kaplan-Meier method and compared between cohorts (Grade 0 vs Grade 1-3) using the log-rank test. To examine the association of transplantation factors with CRS, multivariable models adjusted for potential confounding variables were conducted via logistic regression models. For the multivariate analysis, ordinal outcome applied to proportional odds regression was used to analyzed risk factors for CRS severity.
All P-values were two-sided, and P-value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS v26.0 and R v3.5.2.
Results
Patient characteristics
Sufficient clinical data were available for 287 patients. Nineteen patients were excluded from the analysis for documented infection. Demographic and disease-specific characteristics for the remaining 268 patients were summarized in . 268 patients were divided into 3 cohorts based on the severity of CRS: no CRS (grade 0 CRS; n = 121; 45.1%), grade 1 CRS (n = 87; 32.5%), and grade ≥2 CRS (n = 60; 22.4%). The median follow-up for all patients was 14 months (range, 1–76).
Table 1. Patient characteristics by CRS Grade.
CRS
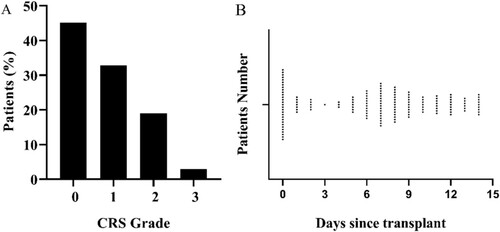
The incidence of CRS was summarized in A. In the 14 days after transplantation, 147/268(54.9%) patients met the criteria for CRS, but most of them were mild (grades 1 or 2) cases. Only eight patients developed severe CRS, and all of them were grade 3 CRS. Nobody received tocilizumab for CRS. There were two time peaks of fever, one is d 0, and the other was near the d+7 (B). Except for fever, other common symptoms were were diarrhea (43.5%), liver dysfunction (35.4%), hypoxia (30.6%), hypotension (29.3%), renal dysfunction (10.9%). Although liver dysfunction, renal dysfunction, and diarrhea were not included in the CRS grade standard, the incidence of renal damage and diarrhea was significantly higher in the CRS grade ≥ 2 cohort than the CRS grade 1 cohort (p = 0.016,0.047, respectively).
Figure 1. The incidence and first time begin fever of CRS after haplo-HCT. (A) Incidence of CRS. (B) Time begin with fever.
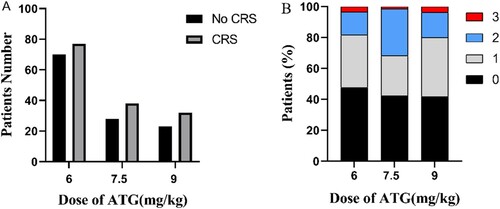
In our study, patients received three doses of ATG (6, 7.5, and 9 mg/kg). Different doses of ATG did not result in significant variance in the occurrence of CRS, showed in A. In addition, we further investigated the incidence of different grades of CRS in patients with the same dose of ATG. At the ATG dose of 7.5 mg/kg, the proportion of grade ≥2 CRS was higher than that of the other two cohorts, but there was no significant difference (B).
Figure 2. The incidence and severity of CRS after haplo-HCT with different doses of ATG. (A) The number of patients who developed CRS or not with different total doses of ATG (6, 7.5, and 9 mg/kg). (B) The percentage of variant CRS grade of patients who applied to different doses of ATG.
On multivariate analysis, age and the disease status at transplantation were risk factor significantly associated with the occurrence of CRS (Odds ratio [OR], 0.282; 95% confidence interval (CI), .143 to .553; P = .000; OR, .977; 95% CI, .957 to .997; p = .021, respectively). However, ordinal logistic regression confirmed that none of the pretransplant characteristics was a significant risk factor for CRS severity.
Impact of CRS on Outcomes after haploidentical transplantation
In our cohort, only six patients failed to achieve neutrophil engraftment; unfortunately, all of them developed CRS. Two events were grade 2 and four events were grade 1. Of 22 patients who did not achieve platelet engraftment, 17/22 patients (77.3%) had CRS including six grade 1 and 11 grade 2. The median time to neutrophil engraftment and platelet engraftment was 11 days and 13 days. In those achieving engraftment, there was no detectable difference in the median time to neutrophil engraftment and platelet engraftment between patients without CRS and with CRS.
Among the 268 patients in the cohort, 156 (58.2%) had at least 1 infection within one year after haplo-HCT. When stratified by CRS grade, 48.8% patients with grade 0, 69.0% patients with grade 1, and 67.7% patients with grade ≥2 developed an infection within one year after haplo-HCT (). In the first one year, patients who developed CRS was associated with a higher incidence of infection significantly (p = .004, not showed). However, there seemed to be no significant relationship between CRS grade and infection rate of different pathogens such as bacterial, CMV, and fungal as showed in .
Table 2. Frequency of infection in the first one year after haplo-HCT, stratified by CRS severity
Univariate analysis revealed that 1-year OS and 1-year DFS were significantly higher in patients with no CRS compared with patients who suffered from CRS (81.20% vs 69.80%, p = .032; 71.60% vs 59.20%, p = .026, respectively) (). Additionally compared with patients without CRS, the patient with CRS had a higher 1-year cumulative incidence of both NRM and relapse (22.9% vs 16.7%, p = .038; 29.0% vs 19.9%, p = 267, respectively). The cumulative incidence of 100-day grades II-IV acute GVHD was 12.4%. However, in terms of cumulative incidence of 100-day grades II-IV acute GVHD and infections, the result did not show significant differences between patients with CRS and no CRS. Moreover, there was no noteworthy deviation between bacterial infection, fungi infection, and virus infection. In the meantime, we analyzed outcomes with the main effect of CRS grade ≥ 2 compared to milder CRS, and the former had significantly lower 1-year OS (64.10% vs 78.00%, p = .009) (Supplementary Table S1). This cohort also had a higher NRM (44.9% at 1 year) and lower DFS (56.4% at 1 year) which did not meet statistical significance potentially because of the small sample.
Table 3. Univariate analysis between patients with CRS and no CRS
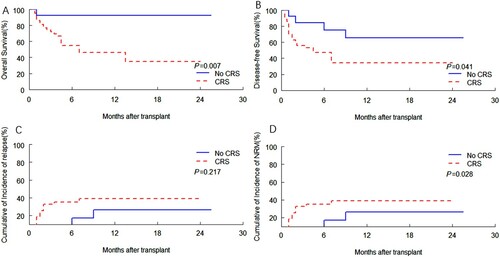
Patients with CRS had significantly lower OS and DFS compared with patients without CRS (p = .009, p = .026, respectively) (A-B), patients with CRS had higher NRM (p = .046) (D). The disease status at transplantation between patients with CRS and patients without CRS were significantly different, so we divided patients into complete remission (CR) cohort and those with uncomplete remission (NR). 103/211(48.8%) patients and 44/57(77.2%) patients developed CRS in the CR cohort and NR cohort, respectively, (p = .000). In addition, we divided the patients into two cohorts for clinical outcome analysis. In the NR cohort, the results were similar to the total recipients (). Patients with CRS had significantly lower OS and DFS compared with patients without CRS (p = .007, p = .041, respectively) (A-B), patients with CRS had higher NRM (p = .028) (D). However, in the CR cohort, there was no significant difference in OS, DFS and NRM between patients with CRS and patients without CRS (Supplementary Figure s1). Patients with CRS had lower OS and DFS compared with patients without CRS (p = .0285, p = .0332, respectively) (Figure 1A-B), which did not reach significant differences.
Figure 3. Kaplan-Meier curves by CRS of association with (A) OS and (B) DFS, and cumulative incidence of (C) relapse and (D) NRM according to CRS. (A) OS. (B) DFS. (C) Relapse. (D) NRM.
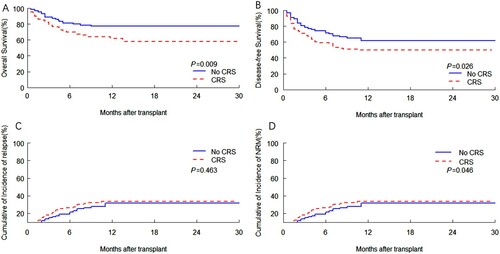
Figure 4. Kaplan-Meier curves by CRS of association with (A) OS and (B) DFS, and cumulative incidence of (C) relapse and (D) NRM according to CRS, among patients limited to NR cohort. (A) OS. (B) DFS. (C) Relapse. (D) NRM.
We further analyzed the causes of death in all patients during the follow-up period, because the OS and NRM of patients with CRS were significantly higher than that of patients without CRS. As showed, relapse was the leading cause of death after haplo-HCT with ATG in our study. Compared with patients without CRS, the percentage of patients who died from infection or GVHD was higher in patients with CRS.
Table 4. The cause of death between patients with CRS and no CRS
Discussion
CRS is a frequent complication described after unmanipulated haploidentical HSCT. However, the majority of published data have been limited to PTCy-based haplo-HCT [Citation15,Citation16,Citation29–32]. In this retrospective study, we reported the development and characteristics of CRS in T-cell replete allogeneic transplant based on ATG. In addition, we analyzed the risk factors related to its development, as well as the impact on posttransplant outcomes.
In our cohort of patients, 147/268 (54.9%) patients developed CRS using ASTCT Consensus Grading for Cytokine Release Syndrome [Citation24]. Among them, 87/147 patients were grade 1, 52/147 patients were grade 2, and only eight patients were grade 3, meeting the criteria for severe CRS. Consistent with previous reports, the clinical manifestations of CRS involved fever, hypotension, respiratory failure, nausea, diarrhea, hepatic failure, and renal failure, tachycardia [Citation33–35]. Although eight patients with severe CRS required vasoactive drugs or oxygen therapy, no one needed intensive-care unit management and no patients received tocilizmab for CRS. Treatment with tocilizumab to block IL-6 signaling pathway had been reported in few cases with severe CRS after haploidentical stem cell infusion with successful resolution of CRS symptoms [Citation16,Citation36]. All the eight patients occurred hypotension, but 6 patients received dopamine to maintain blood pressure. Two patients did not require vasopressors, and the blood pressure was restored after active fluid rehydration. The reason why the two patients were classified to severe CRS was that both of them developed sever hypoxia. They need facemask with oxygen delivered at > 6 L/minute to overcome the oxygenation deficit. In our study, the incidence of CRS was 54.9%, which was markedly lower than prior reports [Citation15,Citation16,Citation28,Citation29,Citation32]. The biggest difference was the use of ATG instead of PTCy for GVHD prophylaxis between our study with others. Salas et al. [Citation17] previously described a lower rate of CRS in haplo-HCT employing dual T-cell depletion with ATG and PTCy. Salas et al showed just 31.3% of patients developed CRS and all of them were grade 1–2. The authors hypothesized that this effect might be explained by the residual effect of ATG, leading to a reduction in the number of alloreactive T cells present in unmanipulated PB allografts, thereby resulting in a lower incidence of CRS [Citation17]. In our analysis, three cohorts of ATG doses of 6 mg/kg, 7.5 mg/kg, 9 mg/kg, the incidence of CRS was respectively 52.4%, 25.8%, 21.8% (p = .668). Furthermore, we found that the incidence rate of different grades of CRS in patients with the same dose of ATG was no significant difference. It seemed that the dose of ATG did not cause an impact on the development of CRS and its severity. In our study, not only the rates of CRS varied from previous reports, but also the time when patients begin pyrexia was not the same. There were two spikes in the duration of fever. We speculated that this might be due to the continuous expansion of T-cells after the reinfusion of stem cells around d+7.
Many studies showed similar results demonstrating that the use of peripheral blood grafts was an independent factor predictive of CRS development after haplo-HCT [Citation29,Citation32,Citation37]. In our series, the source of grafts consisted of PB and PB + BM. However, the incidence of CRS between PB and PB + BM were similar (55.8% verse 44.2%, p = .636). Maude et al. [Citation38] pointed out that severe cytokine-release syndrome was associated with a higher disease burden before infusion. In our analysis, among all the demographic or treatment factors we collected, the disease status at transplantation and age were the risk factors statistically associated with the development of CRS. No factors were significantly associated with development of severe CRS, likely due to the limited number of patients with these events.
In our cohort, all the patients who did not achieve neutrophil engraftment developed CRS. Among all the patients who failed to attain platelet engraftment, 17/22 (77.3%) suffered from CRS. The underlying mechanism between unsuccessful engraftment and CRS was unknown. Preclinical studies showed that specific cytokines encompassing TNFα, IL-4, IL-6, and IL-10, could suppress hematopoiesis [Citation39–41]. In further investigation, the function of cytokine in immune reconstitution is essential.
In our analysis, compared with milder CRS, CRS grade ≥ 2 had significantly lower 1-year OS (64.10% vs 78.00%, p = .009). Nevertheless, CRS grade ≥ 2 was not associated with a statistically higher risk of NRM or grades II to IV acute GVHD. Raj et al. [Citation32] reported that grade ≥ 2 CRS was not associated with statistically significantly grades III to IV acute GVHD. Abboud et al. [Citation16] indicated that the presence of CRS did not alter the rates of aGVHD. However, Solán et al. [Citation29] showed that patients with CRS developed grade II-IV aGVHD more frequently than those without CRS (60% vs 28.6% respectively, P = .012). Perhaps the differences in these results could be attributed to the small sample size in the study cohort as well as the limited number of patients with severe CRS.
Maude et al.[Citation38] reported that a higher disease burden was associated with severe CRS for patients received CAR-T cells targeting CD19. Patients with large tumor burdens appeared to higher incidence and severity of CRS, potentially because this contributed to higher levels of T-cell activation[Citation12]. In our series, 48.8% patients and 77.2% patients developed CRS in the CR cohort and NR cohort, respectively, (p = .000). Thus, we further divided the patients into two cohorts for clinical outcome analysis. When we analyzed the outcomes post-HSCT limited to patients who were unsuccessful in achieving complete remission, patients with CRS had the significantly lower OS and DFS. However, in the CR cohort, there was no significant difference between patients with CRS and without CRS. Even though none of the deaths in our study was directly due to CRS. In our analysis, patients who developed CRS was associated with a higher incidence of infection significantly as showed in . The mechanism of the interplay of disease status might be associated with an increased risk of infection in patients with CRS. It meant that the occurrence of CRS might have long-term effects on the clinical course beyond the early time post-transplantation leading to adverse outcomes, especially in the patients with huge tumor burden.
In our series, the occurrence of CRS caused a grim impact on the outcomes post-transplantation, particularly for patients with a huge disease burden. Besides, the severity of CRS was significantly associated with poor OS. Hence, prevention of CRS is an essential part of future research. A prospective phase II clinical trial using haplo-HCT for patients with active disease acute myeloid leukemia is studying tocilizumab for the prevention of CRS [Citation16]. Prospective studies assessing cytokine levels and their patterns in the peritransplant context may prompt patients who are at higher risk and identify early patients who will develop severe CRS and may benefit from tocilizumab.
The limitations of our study include its retrospective nature, single-center setting, and limited sample size, notably the limited number of severe CRS. Prospective studies are needed to clarify the risk factors involved in CRS development, severity, and the impact of CRS on transplantation outcomes.
Conclusion
In conclusion, we found that CRS appeared less frequently in patients with haplo-HCT based on ATG compared to previous reports about PTCy. In our experience, patients in the NR status at transplantation were inclined to develop CRS and subjected to worse clinical outcomes post haplo-HCT. In our study, severe CRS was uncommon and the symptoms resolved with supportive care. However, the occurrence of CRS was associated with higher NRM and inferior DFS and OS. Prospective clinical trials are needed to provide an appropriate scheme for CRS prophylaxis.
Supplemental Material
Download MS Word (90.7 KB)Acknowledgement
This work was supported by the Chen Xiaoping Foundation for the Development of Science and Technology of Hubei Province. The authors thank the staff from Institute of Hematology of Wuhan Union Hospital for their supports during the study.
Disclosure statement
The authors have no conflict of interest.
Additional information
Funding
References
- Aversa F, Tabilio A, Velardi A, et al. Hematopoietic stem cell transplantation from alternative donors for high-risk acute leukemia: the haploidentical option. Curr Stem Cell Res Ther. 2007;2:105–112. doi:https://doi.org/10.2174/157488807779316973.
- Martelli MF, Reisner Y. Haploidentical ‘megadose’ CD34+ cell transplants for patients with acute leukemia. Leukemia. 2002;16:404–405. doi:https://doi.org/10.1038/sj.leu.2402382.
- Henslee-Downey PJ, Abhyankar SH, Parrish RS, et al. Use of partially mismatched related donors extends access to allogeneic marrow transplant. Blood. 1997;89:3864–3872.
- Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi:https://doi.org/10.1200/jco.2005.09.117.
- Wang Y, Liu D-H, Liu K-Y, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119:978–985. doi:https://doi.org/10.1002/cncr.27761.
- Huang X-J, Liu D-H, Liu K-Y, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006;38:291–297. doi:https://doi.org/10.1038/sj.bmt.1705445.
- Chang YJ, Huang XJ. Haploidentical stem cell transplantation: anti-thymocyte globulin-based experience. Semin Hematol. 2016;53:82–89. doi:https://doi.org/10.1053/j.seminhematol.2016.01.004.
- O’Donnell P, Raj K, Pagliuca A. High fever occurring 4 to 5 days post-transplant of haploidentical bone marrow or peripheral blood stem cells after reduced-intensity conditioning associated with the use of post-transplant cyclophosphamide as prophylaxis for graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:197–198. doi:https://doi.org/10.1016/j.bbmt.2014.10.008.
- McCurdy SR, Muth ST, Tsai H-L, et al. Early fever after haploidentical bone marrow transplantation correlates with class II HLA-mismatching and myeloablation but not outcomes. Biol Blood Marrow Transplant. 2018;24:2056–2064. doi:https://doi.org/10.1016/j.bbmt.2018.06.004.
- Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859–1866. doi:https://doi.org/10.1016/j.bbmt.2012.06.019.
- Powles RL, Kay HEM, Clink HM, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;321:612–615. doi:https://doi.org/10.1016/s0140-6736(83)91793-2.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi:https://doi.org/10.1182/blood-2014-05-552729.
- Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. doi:https://doi.org/10.1182/blood-2014-12-580068.
- Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR t cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi:https://doi.org/10.1038/s41591-018-0041-7.
- Imus PH, Blackford AL, Bettinotti M, et al. Severe cytokine release syndrome after haploidentical peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:2431–2437. doi:https://doi.org/10.1016/j.bbmt.2019.07.027.
- Abboud R, Keller J, Slade M, et al. Severe cytokine-release syndrome after T cell–replete peripheral blood haploidentical donor transplantation is associated with poor survival and anti–IL-6 therapy is safe and well tolerated. Biol Blood Marrow Transplant. 2016;22:1851–1860. doi:https://doi.org/10.1016/j.bbmt.2016.06.010.
- Salas MQ, Lam W, Al-Shaibani Z, et al. Dual T cell depletion with anti-thymocyte globulin and post-transplant cyclophosphamide results in low rates of cytokine release syndrome in peripheral blood haplo-hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:e387–e388. doi:https://doi.org/10.1016/j.bbmt.2019.09.013.
- Arber DA, et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi:https://doi.org/10.1182/blood-2016-03-643544.
- Sullivan KM, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259.
- Przepiorka D, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828.
- Wu Q, et al. Comparison of outcomes of idarubicin intensified TBI–CY and traditional TBI–CY conditioning regimen for high-risk acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation: A single center experience. Leuk Res. 2015;39:1192–1200. doi:https://doi.org/10.1016/j.leukres.2015.08.015.
- Hong M, Wu Q, Hu C, et al. Idarubicin-intensified BUCY2 regimens may lower relapse rate and improve survival in patients undergoing allo-SCT for high-risk hematological malignancies: a retrospective analysis. Bone Marrow Transplant. 2012;47:196–202. doi:https://doi.org/10.1038/bmt.2011.66.
- Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi:https://doi.org/10.1016/j.bbmt.2009.07.004.
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi:https://doi.org/10.1016/j.bbmt.2018.12.758.
- Spitzer TR. Engraftment syndrome: double-edged sword of hematopoietic cell transplants. Bone Marrow Transplant. 2015;50:469–475. doi:https://doi.org/10.1038/bmt.2014.296.
- Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi:https://doi.org/10.1182/blood-2014-01-552984.
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi:https://doi.org/10.1086/339329.
- Patterson TF, Thompson GR, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:433–442. doi:https://doi.org/10.1093/cid/ciw444.
- Solán L, et al. Cytokine release syndrome after allogeneic stem cell transplantation with posttransplant cyclophosphamide. Hematol Oncol. 2020; doi:https://doi.org/10.1002/hon.2772.
- Abid MB, et al. Severity of cytokine release syndrome and its association with infections after T cell-replete haploidentical related donor transplantation. Biol Blood Marrow Transplant. 2020; doi:https://doi.org/10.1016/j.bbmt.2020.06.006.
- Mariotti J, Taurino D, Marino F, et al. Pretransplant active disease status and HLA class II mismatching are associated with increased incidence and severity of cytokine release syndrome after haploidentical transplantation with posttransplant cyclophosphamide. Cancer Med. 2020;9:52–61. doi:https://doi.org/10.1002/cam4.2607.
- Raj RV, Hamadani M, Szabo A, et al. Peripheral blood grafts for T cell–replete haploidentical transplantation increase the incidence and severity of cytokine release syndrome. Biol Blood Marrow Transplant. 2018;24:1664–1670. doi:https://doi.org/10.1016/j.bbmt.2018.04.010.
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi:https://doi.org/10.1056/NEJMoa1215134.
- Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. New Engl J Med. 2006;355:1018–1028. doi:https://doi.org/10.1056/NEJMoa063842.
- Rosenbaum L. Tragedy, perseverance, and chance — the story of CAR-T therapy. N Engl J Med. 2017;377:1313–1315. doi:https://doi.org/10.1056/NEJMp1711886.
- Cho C, Perales MA. Rapid identification of cytokine release syndrome after haploidentical PBSC transplantation and successful therapy with tocilizumab. Bone Marrow Transplant. 2016;51:1620–1621. doi:https://doi.org/10.1038/bmt.2016.229.
- Solh MM, Dickhaus E, Solomon SR. Fevers post infusion of T-cell replete hla mismatched haploidentical hematopoietic stem cells with post-transplant cyclophosphamide: risk factors and impact on transplant outcomes. Bone Marrow Transplant. 2019;54:1756–1763. doi:https://doi.org/10.1038/s41409-019-0522-4.
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi:https://doi.org/10.1056/NEJMoa1407222.
- Broxmeyer HE, et al. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-γ. J Immunol. 1986;136:4487–4495.
- SATO T, MISAGO M, SUKADA J-i, et al. Effect of interleukin-4 on the growth of granulocyte-macrophage progenitor cells stimulated by hematopoietic growth factors. J UOEH. 1990;12:163–174. doi:https://doi.org/10.7888/juoeh.12.163.
- Katayama K, et al. Antagonistic effects of interleukin 6 and G-CSF in the later stage of human granulopoiesis in vitro. Exp Hematol. 1990;18:390–394.
