ABSTRACT
Background
Matrix metalloproteinases (MMPs) play a crucial role in cancer progression and metastasis, however their role in pediatric Acute lymphoblastic leukemia (ALL) is still unrevealed.
Methods
The diagnostic, prognostic and predictive value of tissue inhibitor of metalloproteinase (TIMP-1), MMP-2, MMP-9 and CD34+CD38− cancer stem cells (CSCs) were assessed in bone marrow (BM) samples of 76 ALL children using Flow Cytometry analysis.
Results
There was a significant increase in TIMP-1 [1.52 (0.41–10) versus 0.91(0.6–1.12); respectively, p < 0.001], and CSCs CD34+CD38− [1 (0.03–18.6) versus 0.3 (0.01–1.1), p < 0.001] expression in ALL patients compared to controls. While there were no significant differences regarding MMP-2 and MMP-9 expression between the two groups. The sensitivity, specificity, area under curve (AUC) of MMP-2 were (80.3%, 53.3% and 0.568, p = 0.404), and of MMP-9 were (53.9%, 40% and 0.660, p = 0.053). While that of TIMP-1 were (78.9%, 100% and 0.892, p < 0.001), and that of CD34+CD38− CSCs were (78.9%, 73.3% and 0.855, p < 0.001). Increased TIMP-1 expression associated with the high-risk disease (p < 0.001). CD34+CD38− CSCs and MMP-2 overexpression associated with MRD at day-15, increased BM blast cell count at diagnosis and at day-15 (p < 0.05). TIMP-1 overexpression is associated with shorter DFS and OS rates (p = 0.009 and p = 0.048). Multivariate logistic regression analysis showed that both TIMP-1 [OR: 4.224, p = 0.046], and CD34+CD38− CSCs [OR: 6.873, p = 0.005] could be potential independent diagnostic factors for pediatric ALL.
Conclusion
TIMP-1 and CD34+CD38− CSCs could be possible useful diagnostic markers for pediatric ALL. Also, TIMP-1 is a promising prognostic marker for poor outcome of the patients.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common hematological malignancy in children, and it is a leading cause of cancer-related death in these age-group patients [Citation1]. The ALL is caused by neoplastic transformation of cells of the lymphoid lineage, which leads to accumulation of abnormal, immature and poorly differentiated lymphocytes or rarely natural killer in the BM. These abnormal cells may extend to the peripheral blood, extra-medullary organs, testis and sometimes infiltrate the central nervous system (CNS) [Citation2]. There are 85% of pediatric ALL originates from B-precursors, and the remaining15% are of T-cell origin [Citation3]. Despite the advancement achieved in the diagnosis and treatment strategy for pediatric ALL in the last few years, that leads to improving the survival as well as the cure rates of ALL patients [Citation4]. However, the relapse rate is 15% for childhood B-ALL, and still the prognosis is unfavorable especially for high-risk group ALL patients [Citation5]. The increasing relapse rate could be due to treatment failure which results from the development of multiple drug resistance, or abnormal expression of metabolizing enzymes [Citation6]. Moreover, it had been reported that approximately 20% of ALL children experience relapse in the BM or extra-medullary. This is explained in the phenomenon known as ‘ALL-blast sanctuaries’ or resurgence of blast cells, where the microenvironment changes that surround the blasts niche, and the persistence of MRD might be the key players of relapse [Citation7].
The detection of MRD after treatment is the main predictor of disease relapse in ALL [Citation8]. It is produced by the development of leukemic cells that can resist chemotherapy and consequently mediate treatment failure, in addition to other possible mechanisms like ALL-blast sanctuaries [Citation7]. These leukemic cells can proliferate and survive through their interaction with the BM mesenchymal stromal cells (MSCs), which are the main constituent of BM microenvironment (BMM). This interaction is mediated through the different growth cytokines and growth factors which can support the growth and proliferation of the resistant leukemic cells [Citation8,Citation9]. A growing body of evidence suggests that leukemia stem cells (LSCs) are a subset of cancer cells that maintain chemo-resistance and relapse [Citation10]. These CSCs were reported in acute myeloid leukemia (AML) and chronic myeloid leukemia (CML), but their role in ALL is still unclear [Citation11,Citation12]. Many recent studies reported mutational and phenotypic changes that induce LSC-like properties, which were supported by BMM [Citation12,Citation13]. These phenotypic changes included upregulation of CD34, CD133, P-glycoprotein and BCRP/ABCG2, as well as downregulation of CD38 [Citation12].
The BBM also supports the progression of cancer cells through the BMM-derived proteases such as MMPs [Citation14]. The MMPs is a large family of 24 zinc-dependent endopeptidases that regulate the composition of extracellular matrix (ECM), and have a role in hematopoietic stem cell (HSC) mobilization and function [Citation15]. They can be produced by tumor [Citation16] or stromal cells [Citation17], where they have a critical role in the degradation of ECM components, allowing progression, invasion and metastasis of tumor cells [Citation18]. A unique example of MMPs is MMP-2 and MMP-9 which have a similar catalytic domain among MMPs. They have the fibronectin repeat domain, which can bind and degrade type IV collagen and gelatin. MMP-9 additionally has a type V collagen-like domain [Citation19].
Under normal physiological conditions, the proteolytic activity of MMPs is regulated by the inhibiting activity of the TIMPs, which are formed of four enzymes (TIMP-1 to TIMP-4) [Citation20,Citation21]. Though TIMPs seem to have a protective role in cancer metastasis, however recent reports showed that TIMP-1 overexpression is associated with poor survival and early recurrence in multiple solid tumors including breast and prostate carcinoma [Citation22,Citation23]. On the other hand, TIMP-3 is downregulated in metastatic setting and considered as a tumor suppressor gene [Citation24,Citation25]. Indeed, the role of MMPs in cancer progression is not yet clear especially in ALL, and their deregulation could be a potential target for cancer prognosis and treatment [Citation26]. Therefore, the aim of the current study was to assess the role of MMP-2, MMP-9 and its inhibitor TIMP-1 in pediatric ALL regarding their diagnostic, prognostic and predictive value. In addition, investigate their relation to CD34+CD38− CSCs expression and its implication on cancer progression and outcome. This was performed through correlating TIMP-1, MMP-2, MMP-9 and CD38+CD34− CSCs expression levels with the relevant clinic-pathological features of the patients, survival rates and response to treatment. We hypothesized that this would allow to predict patients’ outcome, and find a potential targeted therapy for those patients, based on the expression levels of MMP-2, MMP-9, TIMP-1 and CD34+CD38− CSCs.
Patients and methods
This prospective cohort study included 76 children with ALL, who were presented to the pediatric outpatients’ clinic, National Cancer Institute (NCI), Cairo University during the period from March 2015 to January 2017. The control group included 16 age and sex-matched healthy individuals who were volunteers for bone marrow transplantation. All patients were subjected to full clinical examination, laboratory work-up, molecular testing, immunophenotyping (IPT) and cytogenetic analysis for confirmation of ALL according to the French-American-British (FAB) and World Health Organization (WHO) criteria [Citation27]. The median follow-up period of the patients was 58.7 (range: 0.23–96.1) months. Patients’ response to treatment was evaluated through morphologic BM examination and IPT for detection of MRD at day 15 and day 42.
Complete remission was defined as achieving; (1) lower than 5% blasts in the BM, (2) Normal maturation of all BM cells, (3) No extra-medullary disease, (4) absolute neutrophil count (ANS) ≥1000/μl, (5) Platelets’ count ≥100,000/μl, (6) Transfusion independent and (7) MRD < 0.01, for at least four weeks.
Relapse was defined by the presence of either BM lymphoblast ≥5%, reappearance of circulating leukemic blasts in the Peripheral blood (PB), development of extra-medullary leukemia and/or MRD ≥ 0.01 [Citation28].
Treatment of the patients
The treatment protocol of the patients is based on total XV St. Jude protocol [Citation29], which consists of three phases: induction, consolidation and continuation. (1) The induction therapy is formed of four-drug regimen (Prednisone, Vincristine, Asparaginase and Daunorubicin), with a subsequent induction treatment consisted of Vincristine, Cyclophosphamide, Cytarabine and Mercaptopurine. On day 42 of induction, MRD and final risk classification were assessed, then the consolidation therapy began. (2) The consolidation phase included four cycles of high-dose methotrexate (HDMTX) with 6mp. (3) The continuation therapy: low-risk patients received daily 6-mercaptopurine and weekly methotrexate with pulses of dexamethasone and vincristine. Two reinduction phases were given between weeks (7–9 and 17–19). Standard-risk patients received pulses of doxorubicin, vincristine and dexamethasone with weekly asparaginase and daily 6-mercaptopurine. They also received two reinduction phases between weeks (7–9 and 17–19). 4) CNS-directed therapy: patients received triple intrathecal chemotherapy (13–18 for low-risk and 16–25 for standard risk), depending on the presenting features and the CNS status. Allogeneic hematopoietic stem-cell transplantation was indicated for high-risk patients (MRD > 1% at the end of induction).
Sample acquisition
The BM aspirates were drawn from all patients by iliac or tibial puncture under complete aseptic conditions. The samples (1 ml) were collected on anticoagulant (EDTA) tubes and processed within few hours for morphologic, molecular and cytogenetic assessment. The IPT was performed on BM blast cells using a comprehensive panel including CD45, CD13, CD33, MHC CLASSII, CD34, CD11C, CD64, CD36, CD14, CD4, CD8, CD7, CD2, CD1, CD56, CD3, CytCD3, CD5, CD19, CD10, CD22, CD20, Cytμ, Cyt CD79α, CD34, CD117 (Bechman Coulter, Miami, U.S.A.), kappa and Lambda (Dako U.S.A., Capinteria, CA).
Assessment of matrix metalloproteinase (MMP-2, MMP-9), TIMP-1 and CD34+CD38− CSCs
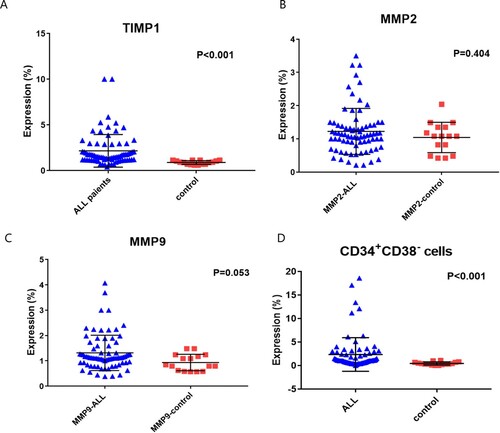
Bone marrow aspiration samples were assessed for MMP-2, MMP-9 and TIMP by Flow Cytometry analysis using anti-human monoclonal antibodies for MMP-9 FITC, MMP-2 PE and TIMP-1 FITC (R&D system, Minneapolis, MN 55413, cat. no. IC9111F, IC9111023P and IC970F; respectively), according to manufactures’ instructions ((A–F)).
Figure 1. Detection of Metrix metalloproteinase (MMP) in pediatric acute lymphoblastic leukemia case. (A): Forwards/side gating on blat population. (B): Fluorescein isothiocyanate (FITC) Isotype control, (C): phycoerythrin (PE) isotype control, (D): MMP-9, (E): Tissue inhibitor of metalloproteinases 1 (TIMP-1) and (F): MMP-2. (G): represent the stem cells gating on the blast population. (H): select the CD34+CD38− population gated on the whole blast population R1.
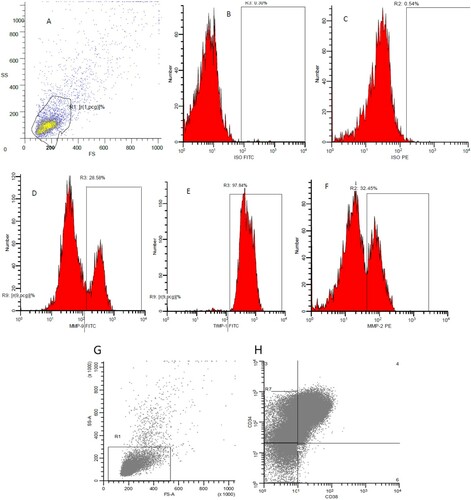
Stem cells were evaluated in the context of CD34+CD38− population gated on CD45 low-expression and CD19+ cells. The whole blood staining method was performed by permeabilization, fixation and intracellular staining using anti-human monoclonal antibodies for CD34 PE-cy5 and CD38 PC7 according to manufacturer’s instructions (Beckman Coulter, Inc., (G,H)).
Viability was done using the 7 AAD test, of which viability of more than 90% was accepted for the analysis. FITC-conjugated anti-mouse IgG2 and PE-conjugated anti-mouse IgG2 isotype-matched control were used as a negative control.
Assessment of the studied markers was performed using Multicolor flow cytometry (Beckman Coulter Navios, U.S.A.), with the acquisition of at least 10,000 events. Proper gating of the malignant population was then created for analysis of the expression of the studied parameters. Analysis was done on Winlist version 6. Flow-cytometer analysis software (Verity, Topsham, ME). The specific median fluorescence intensity (MFI) of each monoclonal antibody used for each sample was calculated by subtracting the background MFI value produced by isotype-matched control from the MFI value generated by the specific antibody.
Statistical analysis
Statistical analysis was performed using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, U.S.A.). Numerical data were expressed as median and range according to the performed normality tests. Qualitative data were expressed as frequency and percentage. The relation between qualitative variables was assessed using Chi-square or Fisher’s exact test as appropriate. Comparison between groups was done using Mann–Whitney test. The Area under the receiver operating curve (ROC) was calculated to investigate the best cutoff value, sensitivity and specificity for the diagnosis of ALL. Survival analysis was done using Kaplan–Meier test. All tests were two-tailed. A p-value < 0.05 was considered significant.
Results
Clinical features of the patients
The current study included 76 patients, of them 47 (61.8%) were males and 29 (38.2%) were females. While males represented 56.3% (9/16) and females represented 43.7% (7/16) of the control group (p = 0.573). The median age of the ALL patients was 6.5 (range: 1–18) years, and that of the control group was 7 (range: 2–12) years (p = 0.629).
The median BM blast at diagnosis was 87% (range; 48 –98%), while it was 0 (range: 0–77%) at day 15 of treatment. The IPT analysis showed that 49 (64.5%) patients had (Pre-B) phenotype, 21 (27.6%) had common B and 6 (7.9%) patients had T-ALL phenotype. Patients were classified according to risk stratification of ALL [Citation29] into low risk (LR) patients in 26 (34.2%), standard risk (SR) in 19 (25.0%) patients and high risk (HR) in 31 (40.8%) patients. There were 22 (30.1%) patients who had MRD < 0.01 at day 15, and 31 (57.4%) patients had MRD < 0.01 at day 42. Complete remission was achieved in 67 (88.2%) patients, and DNA index was favorable (>1) in 10/76 (13.2%) patients ().
Table 1. Clinical characteristics of ALL patients.
Expression levels of TIMP-1, MMP-2, MMP-9 and CSCs CD34+CD38− in ALL patients
There was a significant increase in TIMP-1 expression in ALL patients compared to control subjects [1.52 (0.41–10) versus 0.91(0.6–1.12); respectively, p < 0.001], while there were no significant differences between ALL patients and control group regarding the expression levels of MMP-2 [1.12 (0.21–3.5) versus 1.08 (0.42–2.04), respectively, p = 0.404], and MMP-9 [1.12 (0.38–4.07) versus 0.84 (0.57–1.48), respectively, p = 0.053]. On the other hand, there was a significant increase in CSCs CD34+CD38- in ALL patients [1 (0.03–18.6)] compared to control group [0.3 (0.01–1.1), p < 0.001, ].
Identification of ALL patients using TIMP-1, MMP-2, MMP-9 and CSCs CD34+CD38−
ROC curve analysis was performed to evaluate the diagnostic power of the studied markers to detect ALL patients. The sensitivity, specificity, AUC of MMP-2 were (80.3%, 53.3% and 0.568; respectively) at a cutoff value of 0.841 (p = 0.404), and that of MMP-9 were (53.9%, 40% and 0.660) at a cutoff value of 1.07 (p = 0.053). While the sensitivity, specificity, AUC of TIMP-1 were (78.9%, 100% and 0.892; respectively) at a cutoff value of 1.133 (p < 0.001), and that of CSCs CD34+CD38- were (78.9%, 73.3% and 0.855; respectively) at a cutoff value of 0.55 (p < 0.001, (A,B)). Patients were classified into low-expression and overexpression of the assessed markers according to the appropriate cutoff values obtained by the ROC curve ().
Figure 3. ROC analysis for diagnosis of ALL patients using (A) TIMP-1, MMP-2, MMP-9 and (B) CSCs CD34+CD38−. (C) association between MMP-9/TIMP-1 ratio and complete remission of pediatric ALL patients. (D) Correlation among MMP-2, MMP-9, TIMP-1 and CD34+CD38− cells expressions in the control group. (E) Correlation among MMP-2, MMP-9, TIMP-1 and CD34+CD38− cells expressions in pediatric ALL patients.
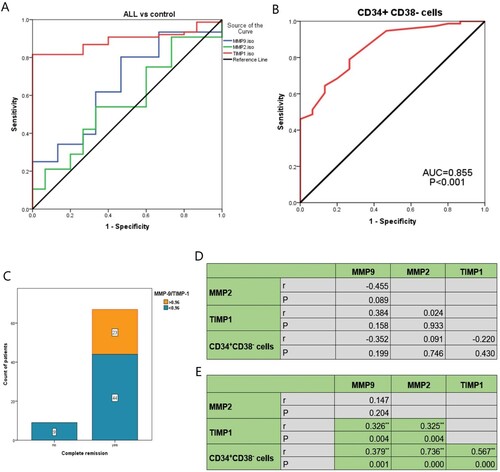
Table 2. ROC curve analysis of TIMP1, MMP2, MMP9 and CD34+CD38− stem cells for identification of ALL patients.
Association between MMP-2, MMP-9, TIMP-1 and CSCs CD34+CD38 expression and the clinical features of the patients
Patients with MMP-2 overexpression showed a significant increase in the BM blast cell count at diagnosis and at day-15 of treatment, as well as a significant increase in MRD at day-15, compared to those with MMP-2 low-expression (p = 0.047, p = 0.001 and p = 0.020; respectively). There was no significant association between MMP-9 expression levels and the assessed relevant clinic-pathological features of the patients ().
Table 3. Association between MMP9, MMP2 and clinical features of the patients.
On the other hand, increased TIMP-1 expression was associated significantly with increased BM blast cell count at diagnosis and at day-15 of treatment (p = 0.033 and p = 0.001; respectively). Also, TIMP-1 was associated significantly with the high-risk stratification of the patients, as out of 60 patients who had TIMP-1 overexpression, 30 (50.0%) patients had high-risk disease, 17 (28.3%) had standard risk and 13 (21.7%) patients had low-risk disease (p < 0.001). There was a significant association between increased TIMP-1 expression and increased MRD at day 15 and day 42 (p < 0.001 for both). Regarding the MMP-9/TIMP-1 ratio, it was observed that all assessed ALL patients who did not achieve complete remission (CR), showed MMP-9/TIMP-1 lower than 0.96, however, this association is of a borderline significance (p = 0.05, (C)).
Regarding CD34+CD38- CSCs, it was associated significantly with increased BM blast cell count at diagnosis and at day-15 of treatment (p = 0.005 and p = 0.003; respectively). Also, it was associated significantly with increased MRD at day-15 (p = 0.015). The CD34+CD38− CSCs overexpression is significantly detected in males rather than in females (p = 0.001), however, this could be explained as a type 1 error due to multiple testing ().
Table 4. Association between TIMP1, CD34+ CD38− and clinical features of the patients.
Correlation among MMP-2, MMP-9, TIMP-1 and CD34+CD38− cells expressions
The present results showed that there was no significant correlation among MMP-2, MMP-9, TIMP-1and CD34+CD38− cells expressions under normal conditions in control subjects ((D)). However, in ALL pediatric patients, there was a significant correlation between TIMP-1 expression and MMP-2 (r = 0.325, p = 0.004), as well as MMP-9 (r = 0.326, p = 0.004). Similarly, CD34+CD38− cells correlated significantly with MMP-2 (r = 0.736, p < 0.001), MMP-9 (r = 0.379, p = 0.001) and TIMP-1 (r = 0.567, p < 0.001, (E)).
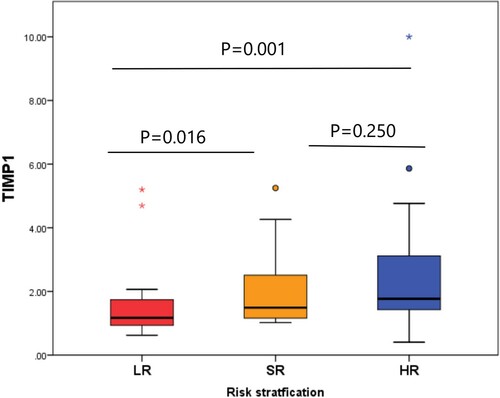
Risk stratification of the patients according to TIMP-1 expression
There was a significant difference in the expression level of TIMP-1 according to the risk stratification of the patients (p = 0.001). Patients with LR disease had a significantly decreased expression of TIMP-1 [1.17 (range; 0.6–5.2)], compared to the SR [1.49 (range; 1–5.3), p = 0.016] and HR patients [1.77 (range; 0.4–10), p = 0.001]. However, there was no significant difference between the SR and HR groups regarding TIMP-1 expression ().
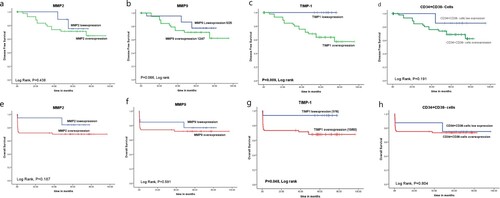
Disease-free survival and overall survival rates of ALL patients
There was a significant association between TIMP-1 overexpression and shorter DFS and OS rates of ALL patients (p = 0.009 and p = 0.048; respectively). However, no significant association was detected between the expression of MMP-2, MMP-9 CD34+CD38− cells and survival (DFS, OS) rates of the patients (p > 0.05, ).
Univariate and multivariate logistic regression analysis for diagnosis of ALL
Univariate analysis revealed that the pediatric ALL associated significantly with increased expression of TIMP-1 [OR: 8.625, p = 0.001], and CD34+CD38− CSCs [OR: 10.312, p < 0.001], while there was no significant association with MMP-2 and MMP-9 expression levels. Multivariate logistic regression analysis showed that both TIMP-1 [OR: 4.224, p = 0.046], and CD34+CD38− CSCs [OR: 6.873, p = 0.005] are independent diagnostic factors for pediatric ALL ().
Table 5. Multivariate logistic regression analysis for the incidence of ALL.
Discussion
The MMPs and their endogenous inhibitors play a crucial role in the invasion and metastasis of solid tumors, however, their role in hematological malignancies especially in ALL is still unrevealed. Moreover, according to the extensive study performed by Kuittinen et al. [Citation30], they concluded that the biological characteristics of MMPs vary between adult and pediatric ALL.
The current study demonstrated a significant increase in TIMP-1 and CD34+CD38− CSCs expression levels in ALL pediatric patients compared to the control group, while there were no significant differences between ALL patients and the control group regarding the expression levels of MMP-2 and MMP-9. However, our data regarding MMP-9 expression are not in agreement with Lin et al. [Citation31], who demonstrated in their study that the expression levels of MMP-9 in the BM of AML and ALL patients were lower than those in the control group, in addition, MMP-9 has no significant influence on patients’ response to treatment. On the other hand, Verma et al. [Citation32], proposed that leukemia cells in B-ALL, may remodel the BMM via the secretion of certain factors inducing the expression of MMP-9, and therefore, increasing tumor’s invasiveness. Indeed, the certain role of MMPs and TIMPs in ALL is not yet clear, especially in the pediatric population who have an extremely a heterogeneous nature of cancer [Citation33]. In an attempt to investigate their diagnostic roles for childhood ALL, the present results demonstrated that TIMP-1 achieved the highest specificity (100%), and AUC (0.892) with a sensitivity of 78.9% for diagnosing pediatric patients with ALL. The TIMP-1 was followed by CD34+CD38− CSCs expression which achieved the same sensitivity as TIMP-1 (78.9%), with a specificity of 73.3% and AUC (0.855). However, MMP-9 and MMP-2 did not show a significant diagnostic power for ALL. Furthermore, these data were confirmed by the multivariate analysis which showed that both TIMP-1 and CD34+CD38− CSCs expression levels are independent diagnostic factors for pediatric ALL.
The current study revealed that patients with MMP-2 overexpression showed a significant increase in the BM blast cell count at diagnosis and at day-15 of treatment, as well as a significant increase in MRD at day-15 compared to those with MMP-2 low-expression. While there was no significant association between MMP-9 expression levels and the assessed relevant clinic-pathological features of the patients including the extra-medullary infiltration, risk stratification of the patients, response to treatment and immunophenotyping of ALL. These data are comparable to that observed by Scrideli et al. [Citation34], who reported a higher expression level of MMP-9 associated significantly with low-risk group patients, and absence of extra-medullary infiltration. In contrast to these results, Schneider et al. [Citation35], demonstrated that MMP-9 was significantly increased in patients with peripheral infiltration than in patients who had no sign of infiltration. This discrepancy in results may be ought to different clinical features, stages, genetic backgrounds and treatment strategies that varied between patients, however further studies are required to validate these data. In line with our results, Schneider et al. [Citation35], found no significant difference between MMP-2, MMP-9 and TIMP-1 expression in T-lineage ALL or B-lineage ALL. However, many studies reported a significant association between MMP-2 and T-ALL phenotype [Citation30,Citation34]. This difference might be due to the small number of patients with T-ALL phenotype included in the current study.
In concordance with our results regarding the MMP-9/TIMP-1 ratio, Scrideli et al. [Citation34], observed no significant association between the MMP-9/TIMP-1 ratio and survival rate or the clinico-pathological features of the patients. However, the present results demonstrated that all assessed ALL patients who did not achieve CR, showed a decreased MMP-9/TIMP-1 lower than 0.96, though this association is of a borderline significance (p < 0.05).
It had been reported that relapsed ALL is the main health problem affecting children with leukemias, and it is indicated by the presence of MRD during therapy [Citation36]. In this context, the present data showed that higher expression levels of MMP-2, TIMP-1 and CD34+CD38− CSCs associated significantly with increased MRD at day-15. Also, they were associated with increased BM leukemia blast cells at diagnosis and at day-15 of treatment. Additionally, increased TIMP-1 expression was associated significantly with MRD at day-42, and with high-risk stratification of the patients, which could be a potential prognostic factor for relapse. Moreover, it had been proved by many recent studies the important role of CD34+CD38− CSCs in ALL relapse and chemo-resistance [Citation37–39].
Furthermore, the current study showed that there was no significant correlation among MMP-2, MMP-9, TIMP-1and CD34+CD38− cells expressions under normal conditions in control subjects. However, in ALL pediatric patients, there was a significant correlation between TIMP-1 expression and MMP-2, as well as MMP-9. Similarly, CD34+CD38− CSCs correlated significantly with MMP-2, MMP-9 and TIMP-1 expression. This observation indicates that there is a disruption occurred in the physiological balance between MMPs and TIMPs, which leads to matrix proteolysis that associated with different pathological diseases including cancer progression and metastasis [Citation33]. Also, the finding of the significant correlation between CD34+CD38− CSCs and the assessed markers could be explained by the data observed by Verma et al [Citation32], who reported that B-ALL cells can cause remodeling in the BMM by stimulating MMP-9 expression in the MSCs through the release of TNFα.
Regarding the survival analysis of the patients, there was a significant association between TIMP-1 overexpression and shorter DFS and OS rates of ALL patients. however, no significant association was detected between the expression of MMP-2, MMP-9, CD34+CD38− cells and DFS or overall survival rates. These results are in agreement with Scrideli et al. [Citation34], who demonstrated that higher expression level of TIMP-1 gene in leukemia cells associated significantly with lower 5-year event-free survival by univariable and multivariable analysis, while there was no significant effect of MMP-9 and MMP-2 on survival rates of the assessed ALL children. Also, Schneider et al. [Citation35], reported no significant impact of surface MMP-9 expression on the overall survival rate of the patients, while its secreted form associated with a lower overall survival rate. On the other hand, many recent published studies reported that MMP-9- positive blast cells associated significantly with the invasive potential of ALL cells, and the poor survival rates of the patients [Citation36,Citation21].
According to the previous discussion, we can conclude that the exact prognostic and predictive role of MMP-2, MMP-9 and TIMP-1 is not well understood, and it is still a debatable issue in the literature. However, our results demonstrated a significant association of MMP-2, TIMP-1 and CD34+CD38− CSCs with MRD, which could be considered potential markers for relapse. Moreover, TIMP-1 expression level showed significant differences according to the patients’ risk. Therefore, it could be used to stratify LR patients from those with SR or HR. Regarding their diagnostic role, the current study provides evidence that TIMP-1 and CD34+CD38− CSCs could be considered as possible diagnostic markers for pediatric ALL. In addition, TIMP-1 is a promising prognostic marker for high-risk disease, short overall and disease-free survival rates and consequently, poor outcome of the patients. However, these are preliminary data and should be validated on a larger number of patients as well as the control group.
Compliance with ethical standards
The study protocol was approved by the ethical committee of NCI, Cairo University, which was in accordance with Helsinki guidelines. Written informed consents were obtained from parents or legal guardians of all participated patients and control subjects before enrollment in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood. 2015;125:3977–3987.
- Shafat MS, Gnaneswaran B, Bowles KM, et al. The bone marrow microenvironment-home of the leukemic blasts. Blood Rev. 2017;31:277–286.
- Pui CH, Behm FG, Singh B, et al. Heterogeneity of presenting features and their relation to treatment outcome in120children with T cell acute lymphoblastic leukemia. Blood. 1990;75(1):174–179.
- Somers K, Evans K, Cheung L, et al. Effective targeting of NAMPT in patient-derived xenograft models of high-risk pediatric acute lymphoblastic leukemia. Leukemia. 2020 Jun;34(6):1524–1539.
- Tran TH, Harris MH, Nguyen JV, et al. Prognostic impact of kinase-activating fusions and IKZF1 deletions in pediatric high-risk B-lineage acute lymphoblastic leukemia. Blood Adv. 2018;2:529–533.
- Huang FL, Liao EC, Li CL, et al. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: Molecular pathways and disease treatments. Oncol Lett. 2020 Jul;20(1):448–454. doi:https://doi.org/10.3892/ol.2020.11583.
- Gaudichon J, Jakobczyk H, Debaize L, et al. Mechanisms of extramedullary relapse in acute lymphoblastic leukemia: reconciling biological concepts and clinical issues. Blood Rev. 2019 Jul;36:40–56.
- Paganin M, Fabbri G, Conter V, et al. Postinduction minimal residual disease monitoring by polymerase chain reaction in children with acute lymphoblastic leukemia. J Clin Oncol. 2014;32:3553–3558.
- Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157.
- Yu Y, Ramena G, Elble RC. The role of cancer stem cells in relapse of solid tumors. Front Biosci. 2012;4:1528–1541.
- Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118:5565–5572.
- Kihira K, Chelakkot VS, Kainuma H, et al. Close interaction with bone marrow mesenchymal stromal cells induces the development of cancer stem cell-like immunophenotype in B cell precursor acute lymphoblastic leukemia cells. Int J Hematol. 2020 Dec;112(6):795–806. doi:https://doi.org/10.1007/s12185-020-02981-z. Epub 2020 Aug 30. PMID: 32862292.
- Brennan L, Narendran A. Cancer stem cells in the development of novel therapeutics for refractory pediatric leukemia. Stem Cells Dev. 2019 Oct 1;28(19):1277–1287. doi:https://doi.org/10.1089/scd.2019.0035.
- Christopherson KW, Cooper S, Hangoc G, et al. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26−/− mice. Exp Hematol. 2003;31:1126–1134.
- Klein G, Schmal O, Aicher WK. Matrix metalloproteinases in stem cell mobilization. Matrix Biol. 2015;44–46:175–183.
- Mehner C, Hockla A, Miller E, et al. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. 2014;5:2736–2749.
- Reggiani F, Labanca V, Mancuso P, et al. Adipose progenitor cell secretion of GM-CSF and MMP-9 promotes a stromal and immunological microenvironment that supports breast cancer progression. Cancer Res. 2017;77:5169–5182.
- Rydlova M, Holubec Jr. L, Ludvikova Jr. M, et al. Biological activity and clinical implications of the matrix metalloproteinases. Anticancer Res. 2008;28:1389–1397.
- Hsiao YH, Su SC, Lin CW, et al. Pathological and therapeutic aspects of matrix metalloproteinases: implications in childhood leukemia. Cancer Metastasis Rev. 2019 Dec;38(4):829–837.
- Kapoor C, Vaidya S, Wadhwan V, et al. Seesaw of matrix metalloproteinases (MMPs). J Cancer Res Ther. 2016;12(1):28.
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174.
- Dos Reis ST, Viana NI, Iscaife A, et al. Loss of TIMP-1 immune expression and tumor recurrence in localized prostate cancer. Int Braz J Urol. 2015;41(6):1088–1095.
- Dechaphunkul A, Phukaoloun M, Kanjanapradit K, et al. Prognostic significance of tissue inhibitor of metalloproteinase-1 in breast cancer. Int J Breast Cancer. 2012;2012:290854.
- Rettori MM, De carvalho AC, Bomfim longo AL, et al. Prognostic significance of TIMP3 hypermethylation in post-treatment salivary rinse from head and neck squamous cell carcinoma patients. Carcinogenesis. 2013;34(1):20–27.
- Jackson HW, Defamie V, Waterhouse P, et al. TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer. 2017 Jan;17(1):38.
- Winer A, Adams S, Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther. 2018 Jun 1;17(6):1147–1155.
- Daniel AA, Attilio O, Robert H, et al. Revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Campana D. Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol. 2009;46(1):100–106.
- Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009 Jun 25;360(26):2730–2741.
- Kuittinen O, Savolainen ER, Koistinen P, et al. MMP-2 and MMP-9 expression in adult and childhood acute lymphatic leukemia (ALL). Leuk Res. 2001 Feb;25(2):125–131.
- Lin LI, Lin DT, Chang CJ, et al. Marrow matrix metalloproteinases (MMPs) and tissue inhibitors of MMP in acute leukaemia: potential role of MMP-9 as a surrogate marker to monitor leukaemic status in patients with acute myelogenous leukaemia. Br J Haematol. 2002 Jun;117(4):835–841.
- Verma D, Zanetti C, Godavarthy PS, et al. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia. 2020 Jun;34(6):1540–1552.
- Kaczorowska A, Miękus N, Stefanowicz J, et al. Selected matrix metalloproteinases (MMP-2, MMP-7) and their inhibitor (TIMP-2) in adult and pediatric cancer. Diagnostics (Basel). 2020 Jul 31;10(8):547.
- Scrideli CA, Cortez MA, Yunes JA, et al. mRNA expression of matrix metalloproteinases (MMPs) 2 and 9 and tissue inhibitor of matrix metalloproteinases (TIMPs) 1 and 2 in childhood acute lymphoblastic leukemia: potential role of TIMP-1 as an adverse prognostic factor. Leuk Res. 2010 Jan;34(1):32–37.
- Schneider P, Costa O, Legrand E, et al. In vitro secretion of matrix metalloprotease 9 is a prognostic marker in childhood acute lymphoblastic leukemia. Leuk Res. 2010 Jan;34(1):24–31.
- Madhusoodhan PP, Carroll WL, Bhatla T. Progress and prospects in pediatric leukemia. Curr Probl Pediatr Adolesc Health Care. 2016 Jul;46(7):229–241.
- Kihira K, Chelakkot VS, Kainuma H, et al. Close interaction with bone marrow mesenchymal stromal cells induces the development of cancer stem cell-like immunophenotype in B cell precursor acute lymphoblastic leukemia cells. Int J Hematol. 2020 Dec;112(6):795–806.
- Brennan L, Narendran A. Cancer stem cells in the development of novel therapeutics for refractory pediatric leukemia. Stem Cells Dev. 2019 Oct 1;28(19):1277–1287. Mudry.
- Mudry RE, Fortney JE, York T, et al. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96:1926–1932.