ABSTRACT
Objectives
Ibrutinib, a potent inhibitor of the Bruton tyrosine kinase, has revolutionized the treatment of many B-cell malignancies. Ibrutinib has an established favorable toxicity profile with up to 8 years of experience in clinical trials; however, despite ibrutinib’s favorable toxicity profile, dose reductions and treatment discontinuations are becoming more evident in clinical practice, particularly in the setting of specific clinical contexts and patient characteristics. This manuscript is set to provide practical recommendations on the management of patients treated with this agent in daily practice.
Methods
A group of multidisciplinary experts from Portugal met to discuss and highlight practical recommendations, supported on both literature and clinical insights, for the management of the treatment with ibrutinib.
Results/discussion
Handling of both toxicities and drug–drug interactions during ibrutinib treatment poses several challenges to healthcare providers and can benefit from a multidisciplinary approach. The involvement of specialties, such as cardiology, infectiology and pharmacology, can bring an added value to patient care, not only in anticipating/managing safety issues and dose adjustments but also in enhancing adherence to treatment, ultimately improving the risk/benefit balance.
Conclusion
By involving a multidisciplinary group of experts, this work provides a set of key recommendations to optimize care and outcomes for ibrutinib-treated patients. Despite not being a fully comprehensive review on the topic, it is intended as a framework to hematologists and other healthcare professionals who manage these patients in their daily clinical practice.
Introduction
Major advances in the understanding of the oncogenic processes of B-cell malignancies have enabled the development and validation of targeted treatment approaches at an ever-increasing rate [Citation1]. The understanding of the dependency of various mature B-cell malignancies on B-cell receptor (BCR) signaling facilitated the development of inhibitors of this pathway, including Bruton tyrosine kinase (BTK) and PI3K-inhibitors. Together with BCL2 antagonists, those targeted agents have been revolutionizing the treatment of certain B-cell malignancies [Citation2], mostly chronic lymphocytic leukemia (CLL) [Citation3], mantle cell lymphoma (MCL) [Citation4] and Waldenström’s macroglobulinemia (WM) [Citation5,Citation6], providing in some circumstances an alternative to chemo-immunotherapy-based strategies.
Ibrutinib, the first BTK inhibitor (BTKi) approved for CLL, MCL and WM treatment in Europe [Citation7,Citation8], is administered orally once-a-day until disease progression or unacceptable toxicity. Although this BTKi is generally well tolerated, a specific side-effect profile has been reported requiring close monitoring to avoid unnecessary toxicities and premature treatment discontinuations that may limit its efficacy [Citation9,Citation10]. This management is particularly challenging among elderly patients who often have multiple comorbidities, are frequently pretreated with immunochemotherapy regimens and are polymedicated, increasing the risk for drug–drug interactions (DDI).
Several international guidelines have addressed the management of patients receiving ibrutinib [Citation9,Citation11–15], but specific evidence and unmet educational needs still persist [Citation7,Citation13,Citation16], namely in the real world and in country-specific settings.
Handling both toxicities and DDI poses several challenges to healthcare providers, but benefits from a multidisciplinary approach. Considering the known safety profile of ibrutinib, the fields of cardiology, infectiology and pharmacology are of great value not only to enhance adherence to treatment but also to manage safety issues and dose adjustments, ultimately improving the risk/benefit balance [Citation17,Citation18].
Having this in mind, a multidisciplinary group of experts, involving hematologists, a cardiologist, an infectious disease specialist and a hospital pharmacist, explored the current challenges related to the management of ibrutinib treatment and the remaining unmet needs in this context. This work reflects our experience and views and provides a set of key recommendations to optimize patient care and outcomes. It is also expected to promote an integrated collaboration among healthcare providers managing ibrutinib-treated patients and is expected to raise awareness about the benefits of a multidisciplinary approach.
Methods
An expert panel of seven Portuguese consultants met to identify pertinent clinical practices, unmet needs and challenges in the management of ibrutinib-treated patients. Four hematologists represented key reference centers in the country, which have considerably rich experience using ibrutinib. A cardiologist, an infectious disease specialist and a pharmacist, all with involvement in the framework of ibrutinib management, complemented this panel. Prior to the experts' meeting, a literature review was conducted to identify the most relevant topics in the field and generate a set of open-ended questions, which were organized in a structured questionnaire. The authors had a pre-meeting to agree on major topics of debate and consolidate the questionnaire. The final set of questions provided context to the work of the expert panel. All experts conducted their independent literature review, considering their expertise and areas of interest. Recommendations within this paper are those of the expert panel and do not strictly follow the summary of product characteristics (SmPC).
Results/discussion
Patient profiling and precautions before initiating treatment with ibrutinib
General considerations
Treatment with ibrutinib should follow the SmPC [Citation8]. It is essential to fully review the list of special warnings and precautions of the SmPC before initiating this treatment, and one should perform a thorough assessment of the patient’s medical history, comorbidities, and concomitant medications (including over-the-counter drugs), as well as prior therapies administered and corresponding responses (including toxicities) [Citation16]. A summary of considerations important to assess before initiating ibrutinib is provided in .
Table 1. Panel recommendations before introducing ibrutinib.
Due to the particular toxicity profile of ibrutinib, certain pre-existing medical conditions deserve special consideration: cardiac-related (including a history of atrial fibrillation – AF, ventricular arrhythmias, congestive heart failure, hypertension and atrial enlargement), bleeding-related (non-immune thrombocytopenia, congenital or acquired coagulopathies, platelet function disorders and history of intracranial or other serious bleeding), and previous or concomitant exposure to conditions and treatments that may increase the risk of infections (particularly corticosteroids and other immunosuppressive agents).
Cardiovascular conditions
A streamlined collaboration between the hematologist and the cardiologist, from the onset, is essential to review pre-existing cardiac conditions and the potential for cardiac-related AEs during treatment with ibrutinib.
AF is common in elderly patients [Citation16,Citation19] and a prior history of AF, or presence of risk factors for developing AF (such as left atrial abnormalities and hypertension), may increase its risk during ibrutinib treatment [Citation20,Citation21]. Although these conditions are not a contraindication for initiating ibrutinib [Citation22,Citation23], existing alternatives should be considered along with a careful risk–benefit evaluation before making this therapeutic decision. We recommend a baseline EKG and cardiac ultrasound to be performed to assess cardiac pre-conditions and its evolution monitored while on ibrutinib, especially in patients with known cardiac dysfunction, providing that treatment initiation is not delayed [Citation24,Citation25]. Given its arrhythmogenic potential, ibrutinib is not recommended in patients with a history of ventricular arrhythmias [Citation26,Citation27]. Smoking cessation, glycemia and cholesterol control are highly recommended in all patients with history of AF to reduce the risk of ischemic events.
In addition, we also recommend to avoid ibrutinib in patients who have mechanical heart valves, as well as in those who require both anticoagulants and antiaggregants [Citation28]. A history of congestive heart failure or atrial enlargement does not absolutely preclude the use of this BTKi but a thorough risk/benefit assessment is again required. Patients with systolic arterial blood pressure ≥160 mm Hg should have their high blood pressure controlled before initiating ibrutinib.
Bleeding history
Although pre-existing hemorrhagic-prone conditions such as thrombocytopenia (<30,000 platelets/mm3), coagulopathies and history of intracranial, or other severe bleeding, do not absolutely preclude the use of ibrutinib, alternative therapeutic approaches should be seriously considered in these populations, and in patients with aneurysms without hemorrhages.
Concomitant medications
Treatment with ibrutinib is not recommended in patients requiring dual antiplatelet therapy due to its known association with bleeding events [Citation15]. The concomitant use of anticoagulants with ibrutinib should be carefully evaluated. Due to scarce data regarding the use of warfarin or other vitamin k antagonists (VKAs), if ibrutinib is chosen in such circumstances, these should be avoided and replaced by a non-VKA direct oral anticoagulants (DOAC) [Citation28]. Patients on anticoagulant therapy, or receiving CYP3A4 inhibitors or inducers, should not be excluded from ibrutinib treatment but should be closely monitored during treatment to prevent DDI. The need for such therapies should be reassessed and discussed with involved medical specialties. Whenever concomitant moderate, or strong, CYP3A4 inhibitors are used, ibrutinib dose should be reduced, respectively, to 280 or 140 mg, or discontinued for a period of less than 7 days, as stated in the SmPC [Citation8].
Use of corticosteroids with ibrutinib should be discouraged, because of the increased risk for infections – fungal, in particular – as recently described [Citation29–31]. Patients with a history of autoimmune conditions should be managed with adequate therapies before initiating treatment with ibrutinib, in line with CLL treatment guidelines [Citation11–13].
Anti-infectious prophylaxis
Patients with B-cell malignancies are at increased risk of infectious morbidity as a result of immunodeficiency, related to the disease itself and to its treatment [Citation16,Citation32]. Therefore, infectious clinical history should be fully reviewed before starting ibrutinib.
We recommend the following serologic tests prior to ibrutinib initiation: herpes simplex virus type 1 (HSV-1), Epstein–Barr virus (EBV), Cytomegalovirus (CMV), toxoplasmosis, human immunodeficiency virus (HIV), hepatitis C virus (HCV) and hepatitis B virus (HBV). Screening for latent tuberculosis is also advisable considering the past and current epidemiological pattern of this infection in Portugal. The need for other serologic tests (e.g. HSV-2, Varicella zoster virus, HAV or syphilis) should be determined on a case-by-case basis depending on factors such as prior therapy, immune status and prior infections.
Vaccination has the potential to prevent or at least decrease the severity and mortality of a number of infections. Before initiating ibrutinib, it is advisable to review patient vaccination schedules and administer the following vaccines: influenza, conjugated pneumococcal vaccine [Citation33], type B Hemophilus influenzae and hepatitis B vaccine in seronegative patients.
Patients with chronic hepatitis B (HBs antigen positive) or occult infection (HBsAg-negative and anti-HBc positive) [Citation34] should be diagnosed with PCR-based HBV-DNA testing and start antiviral prophylaxis with a nucleoside analog (NA) such as entecavir or tenofovir (according to local standard practice), preferably one week prior to initiating ibrutinib and until 12 months after its discontinuation. HBV-DNA testing should be done every 3–6 months during prophylaxis and for at least 12 months after NA withdrawal as HBV reactivations can still occur [Citation13,Citation35]. Acyclovir prophylaxis may be considered in patients with an increased risk of herpetic reactivation or zoster, which is not uncommon in patients receiving ibrutinib [Citation36]. Of note, ibrutinib is known to increase the susceptibility for opportunistic infections, especially pneumocystis and other fungi [Citation37]. Hence, prophylaxis with cotrimoxazole is the preferred option for patients at high risk of infection (history of prior infection, corticosteroids in doses ≥ 20 mg daily for more than 4 weeks, ≥3 prior treatment lines) and in these cases should be administered throughout the entire course of therapy. Prophylaxis is not deemed as essential for treatment-naïve patients, except for the pre-existing conditions described above [Citation16] (e.g. chronic hepatitis B, occult infections). Although no advantages are expected from the widespread use of antibacterial prophylaxis, patients receiving ibrutinib should be closely monitored for fever and neutropenia, and adequate anti-infective therapy introduced, when necessary [Citation38,Citation39]. Patients with an epidemiological risk for strongyloidiasis, such as past travel to Sub-Saharan Africa, Central or South America, even without screening, should receive a course of antiparasitic treatment such as ivermectin – 200 mcg/kg orally once daily for 2 days (some experts recommend repeating the dose 2 weeks apart) or, as an alternative, albendazole 400 mg bid for 7 days – to prevent strongyloides hyperinfection syndrome [Citation40–43].
Monitoring patients on ibrutinib
In general, the surveillance of patients under treatment with ibrutinib is similar between the different B-cell malignancies for which the drug is approved in Europe (CLL, MCL, and WM). However, the incidence of adverse events and discontinuation rates may vary across these conditions, especially due to age and disease characteristics. CLL patients are typically older and have more comorbidities [Citation44], whereas the more aggressive nature of MCL may lead to an increased willingness to manage AEs and better adherence to therapy from both clinicians and patients [Citation45].
Patients receiving ibrutinib should be monitored monthly during the first 6 months, mainly to manage emerging toxicities, and every 3 months thereafter. Depending on pre-existing medical conditions and patient characteristics, a more intensive monitoring may be required during the first month of treatment.
Complete blood count, biochemistry panel (i.e. liver enzymology, renal function) and physical examination should be performed regularly. Patients should be encouraged to frequently monitor blood pressure and report it during routine medical appointments. This will avoid measuring the blood pressure during clinical appointments when anxiety may spuriously raise the values. Some authors recommend EKGs to be performed once every 1 or 2 years during treatment with ibrutinib [Citation16,Citation25] although the chances of capturing the onset of arrhythmic episodes with EKGs are low. Clinicians are encouraged to perform a thorough assessment of specific hemorrhage and cardiac-related symptoms during follow-up of CLL patients treated with ibrutinib, to ensure these events are not underreported.
Patients with two or more prior treatment lines may be more susceptible to infections. Severe fungal infections may occur within the first months of treatment with ibrutinib and should always be considered in the differential diagnosis of infections and specific organ symptoms. These may be difficult to confirm despite the often-pronounced clinical pictures. Notably, opportunistic infections, such as invasive aspergillosis, have been reported in patients with prior/concomitant corticosteroid therapy [Citation46]. Close surveillance is also recommended for patients with prior fungal or CMV infections, or with a history of repeated infections. It is the opinion of the authors that the frequency of laboratory testing for infections should not differ between treatment-experienced and treatment-naïve patients.
Management of adverse events during treatment with ibrutinib
Although ibrutinib is generally well tolerated [Citation47], its toxicity profile includes fatigue, arthralgias, rash, cytopenia, infection, pneumonitis, diarrhea, bleeding, high blood pressure and AF [Citation13,Citation48–54], some of which, depending on their severity, could ultimately lead to treatment discontinuation. It is worth noting that disease-related factors may also be causing some of the abovementioned side effects. The adequate management of ibrutinib’s side effects may prevent unnecessary dose reductions, interruptions, or discontinuations, significantly maximizing its clinical benefits [Citation55]. A long-term safety analysis of the AEs of clinical interest, associated with the use of ibrutinib, is listed in , and the recommendations regarding the management of AEs in ibrutinib-treated patients are summarized in .
Table 2. Proportion of patients with selected AEs of clinical interest with ibrutinib, obtained from pivotal clinical trials and real-world evidence studies (CTCAE ≥ 3).
Table 3. Panel recommendations for monitoring and managing patients on ibrutinib.
Based on the authors’ experience, the type and frequency of AEs observed in the daily practice resembles the safety pattern reported in clinical trials. Still, one study conducted in Portugal with a sample of 68 ibrutinib-treated CLL patients showed that 47% experienced grade≥ 3 AEs and 18% permanently discontinued the treatment after a median follow-up of 12 months [Citation56]. These findings are consistent with other real world data [Citation57,Citation58] and suggest a higher rate of treatment discontinuations than those seen in clinical trials. After 1 year, the RESONATE study in relapsed/refractory (RR) CLL patients [Citation59] reported a discontinuation rate of 7% due to AE/unacceptable toxicity, similar to the 9% in treatment-naïve (TN) patients after 1.5 years (RESONATE-2) [Citation60].
Patient education is an essential component of ibrutinib management and improves adherence to treatment [Citation55]. The alignment between the different stakeholders involved in the care of these patients is essential to ensure an effective patient education. Physicians and pharmacists play a key role in explaining the risk/benefit balance while anticipating the known and most expected adverse events while on ibrutinib, as well as the probable duration of these events. Most toxicities will present predominantly during the first 6 months [Citation9]. Patients should be encouraged to discuss and report any symptoms occurring during ibrutinib treatment so that these may be managed in a timely manner. Signs and symptoms that deserve special attention include palpitations, dizziness, syncope, or even fatigue of unknown cause. The close collaboration between the hematologist and the pharmacist during the first months of treatment is advisable, especially in situations where ibrutinib’s dose adjustment is being weighed.
Management of diarrhea, rash, arthralgias and fatigue
Diarrhea, rash, arthralgia, and fatigue are non-severe AEs commonly associated with ibrutinib which may hamper its clinical benefits, because they may lead to early treatment discontinuation. Diarrhea occurs early during the treatment course, is often transient and unrecurring. Symptomatic treatment with loperamide is usually effective.
Rash can have different clinical presentations. Non-palpable rash (of a late onset after ibrutinib initiation) usually resolves without the need for treatment adjustments or specific therapy. Palpable purpuric rash has an earlier onset and often requires antihistaminic and topic treatments, which can also include corticosteroids for severe cases and even temporary interruptions of ibrutinib [Citation61].
For patients who develop arthralgia, low-dose analgesics should be the first approach (paracetamol, or opioids in case of non-response to paracetamol), along with a more active lifestyle [Citation16]. As for fatigue, it can often be wrongly attributed to advanced age or a coexisting condition. If fatigue persists during ibrutinib treatment, cardiovascular examination is advisable to rule out a cardiac etiology. Patients should be referred to the cardiologist for a complete examination and treatment should be optimized in case of causal cardiac insufficiency. The authors recommend ibrutinib’s dose reduction for severe cases of arthralgia and fatigue that affect daily-life activities, and for which none of the previous strategies were successful, in order to maintain treatment’s adherence and, ultimately, to avoid therapy discontinuation [Citation62].
Management of cardiovascular events
AF (up to 11% [Citation20]) and ventricular arrhythmias (incidence of 0.6% [Citation26]) may develop during ibrutinib treatment, possibly due to off-target effects of the drug [Citation22]. Patients frequently have other cardiac risk factors, including advanced age – a large proportion of patients receiving ibrutinib are 65 years or older – arterial hypertension and other cardiovascular comorbidities [Citation56–58]. Thus, patients with AF who have a CHA₂DS₂-VASc score ≥2, or patients who have other cardiac comorbidities (such as systolic or valvular dysfunctions, recurrent uncontrolled congestive heart failure or left atrial abnormalities) [Citation13] should be consulted with a cardiologist for a comprehensive assessment and risk factor control before starting ibrutinib. Importantly, even though pre-existing cardiovascular risk factors may increase the probability of cardiac AEs during ibrutinib treatment, these can be minimized with adequate management and monitoring. Namely, when the CHA₂DS₂-VASc score is ≥2, safe and effective anticoagulation should be considered. Simultaneously, unjustified use of antiplatelet agents and NSAIDs should be discontinued, and blood pressure rigorously and frequently monitored.
Management of arterial hypertension
De novo or aggravated hypertension may occur in up to 45% of ibrutinib-treated patients [Citation21,Citation44,Citation47]. This condition can easily go undetected and therefore be underreported in daily clinical practice. Notably, hypertension increases over time, both with increasing age and treatment exposure, and shows a steady frequency in longitudinal ibrutinib safety analyses [Citation63,Citation64], increasing its clinical significance. Even though most hypertensive events reported were classified as grade 1 or 2 [Citation63], uncontrolled elevated blood pressure increases the risk of arrhythmias and may ultimately impact myocardial function. Thus, accurately diagnosing and treating this AE may reduce the chance for other major cardiac events [Citation21]. For lowering blood pressure, bisoprolol, a β1-selective beta blocker with cardioprotective properties, may be used. Alternatively, nebivolol (another selective beta blocker) or even carvedilol (with cardioprotective properties) may be considered. Conceivably, antihypertensives should be effective in stabilizing blood pressure, but exceptional cases of uncontrolled hypertension may lead to ibrutinib discontinuation. In a recent study, one out of 205 patients who developed grade 3 or 4 hypertension discontinued ibrutinib [Citation21].
Management of atrial fibrillation
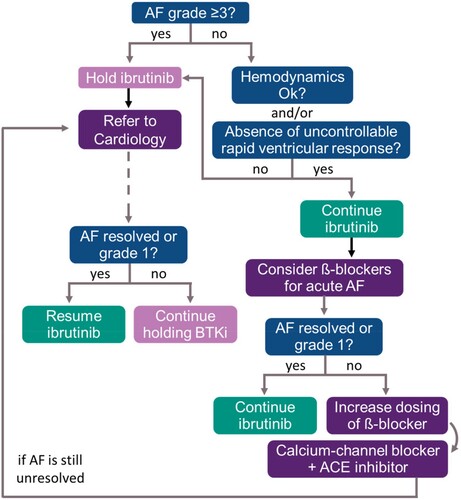
The frequency of AF was increased with ibrutinib when compared to control arms in phase 3 trials and AF events were more common within the first months of treatment [Citation60,Citation65–67] The authors recommend interruption of ibrutinib treatment in the presence of grade ≥3AF (according to the CTCAE) [Citation68] impacting hemodynamics and/or with the rapid ventricular response not controlled with appropriate medications. Notwithstanding, if AF can be controlled with the adequate management and the use of specific anti-arrhythmic therapy, ibrutinib may be reintroduced. Beta-blockers are the preferred choice for managing acute episodes of AF [Citation69–72]. If this strategy is successful in resolving grade ≥3 AF, treatment with ibrutinib can be resumed – unless anticoagulation therapy is required and poses an unacceptable risk of bleeding.
Concomitant use of other channel blockers such as amiodarone, diltiazem, or dronedarone should be avoided due to potential interactions with ibrutinib [Citation16]. However, if treatment with beta-blockers is unsuccessful, even after dose increase, the introduction of calcium-channel blockers is recommended [Citation22] even considering the potential for DDI. Angiotensin converting enzyme (ACE) inhibitors may also be an option [Citation73]. The use of amiodarone is only recommended if there are no therapeutic alternatives [Citation74], during acute AF episodes [Citation69] and in the absence of thyroid and pulmonary-related side effects. A proposed algorithm for AF management is provided in .
Management of anticoagulants and antiplatelets
If anticoagulants are deemed necessary in ibrutinib-treated patients – particularly when the CHA2DS2-VASc score is higher than the HAS-BLED bleeding risk score [Citation9, Citation75] – apixaban (5 mg/day) is the preferred choice, although interaction with CYP3A4 has been reported [Citation7]. Apixaban is one of the anticoagulants with the most favorable safety profile [Citation13] but requires careful monitoring of renal function. In patients who are older than 65 years, weight 60 kg or less, and present creatinine ≥1.5 mg/dL the dose of apixaban can be reduced to 2.5 mg/day. Dabigatran, which has an available antidote, can be an alternative [Citation16], but the absorption levels of this drug may be affected by the effect of ibrutinib on intestinal P-gp [Citation28].
The increased risk of bleeding associated with the use of ibrutinib may discourage the concomitant use of anticoagulants. Physicians should look for alternative tumor treatment approaches in case of inadequate AF control and if the risk/benefit ratio requires ibrutinib’s discontinuation, especially in patients with CHA2DS2-VASc scores ≥2 [Citation28].
Regarding the use of antiplatelets, the need for aspirin should be carefully assessed and discussed with the cardiologist. Antiplatelets are required in patients with a history of acute myocardial infarction in the previous year, or cardiac stent surgery within the last 6 months. Replacing aspirin for triflusal may be an appropriate option in patients initiating ibrutinib [Citation76]. Nonetheless, aspirin discontinuation comes with a risk since ibrutinib’s antiplatelet effect may not be sufficient. In patients with a high-risk profile (prior stroke or heart surgery), combining ibrutinib with an antiplatelet drug, when no alternatives to ibrutinib exist, may prove to be an adequate strategy [Citation28]. This complex scenario requires the compulsory involvement of the cardiologist and a consensus between specialties, to decide on the best strategy to treat patients [Citation15].
Management of bleeding events
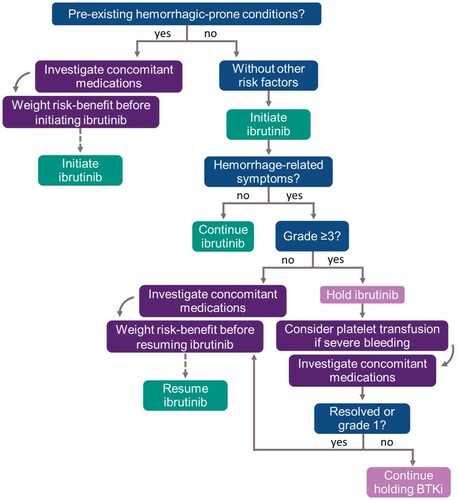
The management of bleeding events should consider the grading of the event and patient characteristics. Hemorrhagic risk is frequently multifactorial. In the event of life-threatening bleeding (grade ≥3) antiplatelet drugs should be stopped and ibrutinib withheld until event resolution. Platelet transfusion should be considered in the cases of severe bleeding. Warfarin is not recommended with ibrutinib since it is associated with major bleeding events [Citation7]. In mild to moderate bleeding, ibrutinib dose reduction, or treatment interruption, may be considered for a short period. A proposed algorithm for evaluating hemorrhagic risk and for managing bleeding events is provided in .
Management of infections
As previously stated, cases of infections have been reported in patients treated with ibrutinib, more frequently so in the earlier course of treatment (first 6 months), and decreasing with time [Citation16,Citation77]. The incidence of infections is higher in relapsing or refractory patients or in the presence of other risk factors such as steroid and rituximab treatment, neutropenia and pre-existing hypogammaglobulinemia [Citation46,Citation78].
Patients receiving ibrutinib should be monitored for fever, neutropenia and infection, and a complete investigation and early treatment should be undertaken considering common and emergent agents. In the event of severe infections (grade ≥3) or related complications, ibrutinib treatment should be withheld and the causative agent(s) of infection determined. Upon infection resolution, ibrutinib should be reintroduced at an adequate dose [Citation16,Citation28].
Early investigation and antifungal pre-emptive or empiric therapy should be considered for any infected patient on ibrutinib [Citation38,Citation39]. Although the expected impact on T-cell function is low, different opportunistic fungal infections have been sporadically reported in patients treated with ibrutinib, including cryptococcosis, Pneumocystis (jirovecii) pneumonia (PJP), histoplasmosis, invasive aspergillosis, mucormycosis and disseminated fusariosis in heavily pretreated patients [Citation79,Citation80]. Therefore, it is essential to investigate the possible presence of these agents, as well as some rare infections such as disseminated viral or mycobacterial infections. Of note, it is important to maintain a high index of suspicion regarding aspergillosis in ibrutinib-treated patients with risk factors such as old age, advanced disease, concurrent corticosteroids, prior immunosuppression, diabetes and liver disease. In more complex cases, a close surveillance for fungal infections should be done according to the patients’ risk. After a complete diagnosis workup, pre-emptive amphotericin B can be considered in selected cases, until the availability of the microbiological results.
Antifungal therapeutic options are limited since all azoles are strong inhibitors of the CYP3A4 and are not generally recommended in combination with ibrutinib. These drug interactions are of particular concern because the recommended course of therapy for fungal infections is prolonged. After the induction phase of antifungal treatment both therapies can be maintained with a lower dose of ibrutinib, and with careful clinical monitoring for toxicity. Pharmacological alternatives may however be considered.
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease caused by the reactivation of John Cunningham virus (JC virus) and a life-threatening complication occasionally associated with the use of ibrutinib [Citation81]. The onset of neurologic symptoms in ibrutinib-treated patients should prompt clinical suspicion and early treatment discontinuation, followed by appropriate diagnostic assessment.
Low immunoglobulin levels are common in CLL, and other B-cell malignancies, as a result of the natural history of disease and prior treatments. Hypogammaglobulinemia should be identified and reposition must be considered, in cases of secondary immunodeficiencies, for patients experiencing severe or recurrent infections, when antimicrobial treatment is not effective and specific antibody deficiency or serum IgG level of <4 g/L are identified [Citation82].
As for common antimicrobials therapies, those can be prescribed. Although clarithromycin and ciprofloxacin are commonly used, both agents are CYP3A4 inhibitors, which may intensify ibrutinib levels, and increase toxicity.
The role of the pharmacist in the management of ibrutinib
Pharmacists play a key role in expanding and enhancing the care of patients treated with ibrutinib, given their regular and close contact with these patients (). This is particularly important in the long run as patients tend to be less proactive in reporting new medications, and to forget the complexity and recommendations regarding the use of ibrutinib (e.g. adequate dosage, routes of administration and required actions when toxicity emerges).
Table 4. Panel Recommendations from pharmacist perspective.
The regular follow-up by the pharmacist allows the identification of concomitant medication with potential for DDI, which may not have been reported by the patient to the clinicians. The potential for DDIs should be cautiously evaluated considering the high prevalence of comorbidities in the elderly patient population where polypharmacy is common and challenging. After 6 months of treatment, the pharmacist should continue to track the patient’s adherence to the treatment plan, while always reviewing the potential for DDI. Notwithstanding, whenever patient compliance with the daily oral treatment becomes a limiting factor, treatments alternatives should be considered.
Dietary counseling should not be overlooked in the management of ibrutinib-treated patients. Fish or flaxseed oils should not be used concomitantly with ibrutinib as these can increase the risk of bleeding. Grapefruit, pomegranate, St. John's wort and bitter orange should not be consumed since all these can interfere with ibrutinib levels [Citation13].
In summary, the pharmacist has the responsibility to promote education and empower patients to play an active role in dealing with the disease by expressing their views, preferences and needs, converging to a more patient-centered care [Citation83].
Conclusions
Ibrutinib has shown clear benefits in the treatment of B-cell malignancies at different disease phases. Since ibrutinib’s first approval, in 2014, for CLL, thousands of patients have been treated, providing a clear picture of the particular toxicity profile of this drug. The management of cardiovascular and hemorrhagic side effects, infections and DDI requires a skilled approach, which greatly benefits from a multidisciplinary team. It is essential to mitigate toxicities, treat adverse effects and carefully manage dose reductions and interruptions in order to maximize ibrutinib’s clinical benefits.
We recognize the need for developing a decision algorithm and an action plan, based on pre-existing conditions, for timely referrals to cardiology and infectious disease specialists. The support of hospital pharmacists and careful patient education is of great value, and even though the patient’s journey may vary according to the characteristics of the hospital/health care unit involved, some basic principles are common.
With this work, we aimed at generating a set of recommendations for healthcare providers who manage patients treated with ibrutinib, emphasizing the importance of a multidisciplinary approach and a close proximity between specialties, which ultimately leads to optimized practices and improved patient outcomes.
Acknowledgments
The authors would like to thank Diogo Morais from Clinical Trials & Consulting Services, for providing medical writing and editorial support to this manuscript.
Disclosure statement
The authors report the following conflict of interest: JC provided consultancy and participated in advisory boards from Janssen, Gilead Sciences, BMS, Abbvie, Takeda, Sanofi, Travel support from Roche, Janssen, Gilead Sciences, Abbvie, and non-remunerated activities (consultancy and teaching) for Janssen. JMM provided consultancy and participated in advisory boards from Janssen, Gilead, BMS, Abbvie, Roche, Celgene, Takeda, Pfizer, Novartis, MSD, Incyte, Servier, Astellas, AstraZeneca, Travel support from Gilead, BMS, Celgene, Roche, Novartis. PM is an ETNA-AF investigator, and has received lecture and research fees from Daiichi-Sankyo, Bayer, Boehringer Ingelheim and Pfizer/BMS. JR provided consultancy and participated in advisory boards from Janssen, Abbvie, Takeda, Gilead, Astra Zeneca and Evigrade. MGS received research grants from Gilead Sciences and Astrazeneca, provided consultancy and participated in advisory boards from Janssen, Gilead Sciences, BMS, Abbvie, Roche, MSD, Takeda, Novartis and ADC Therapeutics and participated in non-remunerated activities (consultancy and teaching) for Roche, Celgene, Takeda, Abbvie, BMS, MSD, Pfizer, Janssen, Takeda and received Travel support from Celgene, Roche, Janssen, Gilead Sciences, MSD, Takeda, Abbvie. LS and HMG report no conflict of interests.
Additional information
Funding
Notes on contributors
José Pedro Carda
José Pedro Carda (MD, MsC) is a Hematologist, with a Masters in Medicine from the University of Coimbra, being a PhD student in the same institution. He is an Assistant Professor at University of Coimbra, and a Hematologist in the Hematology Department, Coimbra University Hospital (CHUC), Portugal since 2007. He is also a researcher at Coimbra Institute for Clinical and Biomedical Research (iCBR). Since 2017, he is the Secretary of the Portuguese Hematology Society. JPC has a solid publication track record and is actively involved in clinical research.
Lurdes Santos
Lurdes Santos (MD, PhD) is an Infectious disease specialist, with a medicine degree and a PhD in Medicine from the Faculdade de Medicina - Universidade do Porto. LS was also awarded the specialty in infectious diseases and intensive medicine from the Portuguese Medical Association (Ordem do Médicos). LS is also the coordinator of the Intensive Care unit for infectious diseases and the coordinator for scientific meetings at the Centro Hospitalar Universitário de São João (Porto, Portugal) since 2001 and 2005, respectively. LS is also a Professor at the Faculdade de Medicina - Universidade do Porto since 2006, and a Principal Researcher at the Nephrology and Infectious Diseases R&D, I3S - Instituto de Investigação e Inovação em Saúde, Universidade do Porto (Porto – Portugal). LS has over 40 publications in peer-reviewed journals and an extensive scientific and research track record.
José Mário Mariz
José Mário Mariz (MD) is an Hematologist, with a degree in Medicine from the Faculty of Medicine of the University of Porto in 1990. JMM is a specialist in Clinical Hematology since 1998 and a member of the Pharmacy and Therapeutics Commission at IPO Porto between 2000 and 2002. In 2001 and 2002 worked as a teacher in Hematology of the Pharmaceutical Sciences Course at Instituto Superior de Ciências da Saúde Norte – CESPU and a guest lecturer in the post-graduate course at the Faculty of Medicine of the University of Porto from 2011 to the present date. JMM is the Director of the OncoHematology Department of the IPO Porto since 2006 to the present date. Also a member of Infarmed’s Health Technology Assessment Committee (CATS) since 2013 and a Senior Assistant in Clinical Hematology since 2015. He has extensive clinical research experience involving different drugs, in different hematological diseases, as National Coordinator, Principal Investigator and Co-investigator.
Pedro Monteiro
Pedro Monteiro (MD, PhD) is a cardiologist with a degree and a PhD in Medicine from the Faculdade de Medicina da Universidade de Coimbra. PM have been highly involved in research since 2001, both in Basic and in Clinical Research fields. Regarding Basic Research, PM studied the effect of several marketed therapeutic agents on cellular and molecular mechanisms underlying global myocardial ischemia/reperfusion in a rodent model of non-obese type 2 diabetes. Currently more focused in Clinical Research, studying the link between changes in glucose metabolism and clinical features of coronary heart failure, as well as their possible epidemiological, clinical, therapeutic and diagnosis implications. Currently, my interests in this field include the study of global cardiovascular risk, myocardial ischemia, intensive care in cardiology, heart failure and atrial fibrillation. As the Head of the Clinical Research in Cardiology Unit, at Centro Hospitalar e Universitário de Coimbra, is currently highly enrolled also in numerous ongoing multicenter Phase III clinical trials (including those evaluating the cardioprotection by the human monoclonal anti-PCSK9 antibody, Evolocumab – FOURIER and EBBINGHAUS), either as National Coordinator and/or Principal Investigator.
Humberto Miguel Gonçalves
Humberto Miguel Gonçalves (PharmD) is a pharmacist with extensive research experience in hospital pharmacy. HMG currently works at the Serviço Farmacêutico, Instituto Português de Oncologia de Lisboa, Francisco Gentil (Lisbon, Portugal).
João Raposo
João Raposo (MD) is a Hematologist with an extensive clinical experience. JR is currently the Director of the Serviço de Hematologia e Transplante de Medula, Centro Hospitalar Universitário Lisboa Norte, EPE (Lisbon, Portugal).
Maria Gomes da Silva
Maria Gomes da Silva (MD, PhD) is senior consultant in Hematology with a degree in Medicine from the Faculdade de Medicina - Universidade de Lisboa (Lisbon, Portugal) and a PhD in Medicine from the NOVA Medical School. MGS is head of the Hematology Unit at the Instituto Português de Oncologia de Lisboa, Francisco Gentil, EPE (Lisbon, Portugal), and professor of Hematology at the NOVA Medical School. She has an extensive publication and clinical research track record with more than 70 publications in peer-review journals, participation in a myriad of clinical research projects and is a member of several international scientific societies and European lymphoma cooperative study groups.
References
- Bosch F, Dalla-Favera R. Chronic lymphocytic leukaemia: from genetics to treatment. Nat Rev Clin Oncol. 2019;16(11):684–701. doi:https://doi.org/10.1038/s41571-019-0239-8
- Castillo JJ, Treon SP, Davids MS. Inhibition of the Bruton tyrosine kinase pathway in B-cell lymphoproliferative disorders. Cancer J. 2016;22(1):34–39. doi:https://doi.org/10.1097/PPO.0000000000000170
- Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207–1213. doi:https://doi.org/10.1182/blood-2013-07-515361
- Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Publ Gr. 2014;14; doi:https://doi.org/10.1038/nrc3702
- Zanwar S, Abeykoon JP, Kapoor P. Current therapeutic options in Waldenström macroglobulinemia. Oncol Hematol Rev. 2019;15(1):39-47. doi:https://doi.org/10.17925/OHR.2019.15.1.39.
- Abeykoon JP, Yanamandra U, Kapoor P. New developments in the management of Waldenström macroglobulinemia. Cancer Manag Res. 2017: 9–73. doi:https://doi.org/10.2147/CMAR.S94059
- Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133(12):1298–1307. doi:https://doi.org/10.1182/blood-2018-11-846808
- Ibrutinib – Annex I summary of product characteristics. [cited July 5, 2019]. https://www.ema.europa.eu/en/documents/product-information/imbruvica-epar-product-information_en.pdf
- De Weerdt I, Koopmans SM, Kater AP, et al. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102(10):1629–1639. doi:https://doi.org/10.3324/haematol.2017.164103
- Orellana-Noia VM, Kluin-Nelemans JC, Williams ME. Front-line therapy in elderly patients with mantle cell lymphoma. Ann Lymphoma. 2019;3:8–8. doi:https://doi.org/10.21037/aol.2019.06.02
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(Suppl 5(February)):v78–v84. doi:https://doi.org/10.1093/annonc/mdv303
- ESMO Guidelines CommitteeChronic lymphocytic leukaemia treatment recommendations. eUpdate published online 27 June 2017. Ann Oncol. 2017;28(suppl_4):iv149–iv152. doi: https://doi.org/10.1093/annonc/mdx242.
- Brown JR. How i treat CLL patients with ibrutinib. Blood. 2018;131(4):379–386. doi:https://doi.org/10.1182/blood-2017-08-764712
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;32(1):23–33. doi:https://doi.org/10.1016/j.annonc.2020.09.019
- Shatzel JJ, Olson SR, Tao DL, et al. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15(5):835–847. doi:https://doi.org/10.1111/jth.13651
- Gribben JG, Bosch F, Cymbalista F, et al. Optimising outcomes for patients with chronic lymphocytic leukaemia on ibrutinib therapy: European recommendations for clinical practice. Br J Haematol. 2018;180(5):666–679. doi:https://doi.org/10.1111/bjh.15080
- Thompson PA, Levy V, Tam CS, et al.. The impact of atrial fibrillation on subsequent survival of patients receiving ibrutinib as treatment of chronic lymphocytic leukemia (CLL): an international study 2016;128(22):3242–3242. doi.org/https://doi.org/10.1182/blood.V128.22.3242.3242.
- Finnes HD, Chaffee KG, Call TG, et al. Pharmacovigilance during ibrutinib therapy for chronic lymphocytic leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) in routine clinical practice HHS public Access author manuscript. Leuk Lymphoma. 2017;58(6):1376–1383. doi:https://doi.org/10.1080/10428194.2016.1251592
- Shanafelt TD, Parikh SA, Noseworthy PA, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2017;58(7):1630–1639. doi:https://doi.org/10.1080/10428194.2016.1257795
- Mato AR, Clasen S, Pickens P, et al. Left atrial abnormality (LAA) as a predictor of ibrutinib-associated atrial fibrillation in patients with chronic lymphocytic leukemia. Cancer Biol Ther. 2018;19(1):1–2. doi:https://doi.org/10.1080/15384047.2017.1394554
- Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919–1928. doi:https://doi.org/10.1182/blood.2019000840
- Ganatra S, Sharma A, Shah S, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4(12):1491–1500. doi:https://doi.org/10.1016/j.jacep.2018.06.004
- Wiczer TE, Levine LB, Brumbaugh J, et al. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1(20):1739–1748. doi:https://doi.org/10.1182/bloodadvances.2017009720
- Reda G, Fattizzo B, Cassin R, et al. Predictors of atrial fibrillation in ibrutinib-treated CLL patients: a prospective study. J Hematol Oncol. 2018;11(1). doi:https://doi.org/10.1186/s13045-018-0626-0
- Baptiste F, Cautela J, Ancedy Y, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Hear. 2019;6(1):1–9. doi:https://doi.org/10.1136/openhrt-2019-001049
- Guha A, Derbala MH, Zhao Q, et al. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72(6):697–698. doi:https://doi.org/10.1016/j.jacc.2018.06.002
- Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129(18):2581–2584. doi:https://doi.org/10.1182/blood-2016-10-742437
- Stühlinger MC, Weltermann A, Staber P, et al. Recommendations for ibrutinib treatment in patients with atrial fibrillation and/or elevated cardiovascular risk. Wien Klin Wochenschr. 2019. doi:https://doi.org/10.1007/s00508-019-1534-1
- CHMP. Committee for Medicinal Products for Human Use (CHMP) CHMP Assessment Report; 2014.
- Varughese T, Taur Y, Cohen N, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;67(5):687–692. doi:https://doi.org/10.1093/cid/ciy175
- Maffei R, Maccaferri M, Arletti L, et al. Immunomodulatory effect of ibrutinib: reducing the barrier against fungal infections. Blood Rev. 2019:100635. doi:https://doi.org/10.1016/j.blre.2019.100635
- Morrison V. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clin Lymphoma Myeloma. 2009;9(5):365–370. doi:https://doi.org/10.3816/CLM.2009.n.071
- Andrick B, Alwhaibi A, DeRemer DL, et al. Lack of adequate pneumococcal vaccination response in chronic lymphocytic leukaemia patients receiving ibrutinib. Br J Haematol. 2018;182(5):712–714. doi:https://doi.org/10.1111/bjh.14855
- Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:https://doi.org/10.1016/j.jhep.2017.03.021
- Law MF, Ho R, Cheung CKM, et al. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies treated with anticancer therapy. World J Gastroenterol. 2016;22(28):6484–6500. doi:https://doi.org/10.3748/wjg.v22.i28.6484
- Kreiniz N, Bejar J, Polliack A, et al. Severe pneumonia associated with ibrutinib monotherapy for CLL and lymphoma. Hematol Oncol. 2018;36(1):349–354. doi:https://doi.org/10.1002/hon.2387
- Ahn IE, Jerussi T, Farooqui M, et al. Atypical pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128(15):1940–1943. doi:https://doi.org/10.1182/blood-2016-06-722991
- Reinwald M, Silva JT, Mueller NJ, et al. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24:S53–S70. doi:https://doi.org/10.1016/j.cmi.2018.02.009
- Maschmeyer G, De Greef J, Mellinghoff SC, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European conference on infections in leukemia (ECIL). Leukemia. 2019;33(4):844–862. doi:https://doi.org/10.1038/s41375-019-0388-x
- La Hoz RM, Morris MI. Intestinal parasites including cryptosporidium, cyclospora, giardia, and microsporidia, entamoeba histolytica, strongyloides, schistosomiasis, and echinococcus: Guidelines from the American Society of Transplantation Infectious Diseases Community of Pract. Clin Transplant. 2019;33(9):1–16. doi:https://doi.org/10.1111/ctr.13618
- Fardet L, Généreau T, Poirot J-L, et al. Severe strongyloidiasis in corticosteroid-treated patients: case series and literature review. J Infect. 2007;54(1):18–27. doi:https://doi.org/10.1016/j.jinf.2006.01.016
- Requena-Méndez A, Buonfrate D, Gomez-Junyent J, et al. Evidence-based guidelines for screening and management of strongyloidiasis in non-endemic countries. Am J Trop Med Hyg. 2017;97(3):645–652. doi:https://doi.org/10.4269/ajtmh.16-0923
- Silva-Pinto A, Rocha-Pereira N, Andrade J, et al. Protocolo de prevenção de infeções relacionadas com o tratamento de neoplasias hematológicas. Acta Med Port. 2018;31(6):347. doi:https://doi.org/10.20344/amp.10035
- Byrd JC, Furman RR, Coutre SE, et al. 2020. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. doi:https://doi.org/10.1158/1078-0432.ccr-19-2856
- Rule S, Dreyling MH, Goy A, et al. Long-Term outcomes with ibrutinib versus the prior regimen: A pooled Analysis in relapsed/refractory (R/R) mantle cell Lymphoma (MCL) with up to 7.5 years of extended follow-up. Blood. 2019;134(Supplement_1):1538–1538. doi:https://doi.org/10.1182/blood-2019-124691
- Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–1959. doi:https://doi.org/10.1182/blood-2017-11-818286
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi:https://doi.org/10.1056/NEJMoa1812836
- Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. doi:https://doi.org/10.1182/blood-2014-10-606038
- O’Brien S, Hillmen P, Coutre S, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma, Myeloma Leuk. 2018;18(10):648–657.e15. doi:https://doi.org/10.1016/j.clml.2018.06.016
- Hallek M, Kay NE, Osterborg A, et al. The HELIOS trial protocol: a Phase III study of ibrutinib in combination with bendamustine and rituximab in relapsed/ refractory chronic lymphocytic leukemia. doi:https://doi.org/10.2217/FON.14.119
- Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94. doi:https://doi.org/10.1200/JCO.2012.42.7906
- Vitale C, Ahn IE, Sivina M, et al. Autoimmune cytopenias in patients with chronic lymphocytic leukemia treated with ibrutinib. 101; 2016. doi:https://doi.org/10.3324/haematol.2015.138289
- Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129(19):2612–2615. doi:https://doi.org/10.1182/blood-2016-12-737346
- Itchaki G, Brown JR. Experience with ibrutinib for first-line use in patients with chronic lymphocytic leukemia. Ther Adv Hematol. 2017;9(1):3–19. doi:https://doi.org/10.1177/2040620717741861
- Ysebaert L, Quinquenel A, Bijou F, et al. Overall survival benefit of symptom monitoring in real-world patients with chronic lymphocytic leukaemia treated with ibrutinib: a FiLO group study. Eur J Cancer. 2020;135:170–172. doi:https://doi.org/10.1016/j.ejca.2020.05.016
- Silva S, Melo JA, Espada E, et al. Ibrutinib Na vida real: experiencia multicêntrica November Algarve, Portugal Portuguese Society of Hematology Annual Meeting 2017.
- Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879. doi:https://doi.org/10.3324/haematol.2017.182907
- Winqvist M, Andersson PO, Asklid A, et al. Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30-month follow up of the Swedish compassionate use cohort. Haematologica. 2019;104(5):e208–e210. doi:https://doi.org/10.3324/haematol.2018.198820
- Brown JR, Hillmen P, O’Brien S, et al. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATETM trial of ibrutinib versus Ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Blood. 2014;124(21):3331–3331. doi:https://doi.org/10.1182/blood.v124.21.3331.3331
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi:https://doi.org/10.1056/NEJMoa1509388
- Iberri DJ, Kwong BY, Stevens LA, et al. Ibrutinib-associated rash: a single-centre experience of clinicopathological features and management. Br J Haematol. 2018;180(1):164–166. doi:https://doi.org/10.1111/bjh.14302
- Dasanu CA. Severe arthritic syndrome due to ibrutinib use for chronic lymphocytic leukemia. J Oncol Pharm Pract. 2019;25(4):1003–1005. doi:https://doi.org/10.1177/1078155218772327
- Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;2019. doi:https://doi.org/10.1002/ajh.25638
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798. doi:https://doi.org/10.1038/s41375-019-0602-x
- Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi:https://doi.org/10.1056/NEJMoa1400376
- Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. doi:https://doi.org/10.1016/S1470-2045(15)00465-9
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–778. doi:https://doi.org/10.1016/S0140-6736(15)00667-4
- Cancer Therapy Evaluation Program (CTEP). 2017. Common terminology criteria for adverse events (CTCAE).v.5.0[5-7]. Cancer Ther Eval Progr.
- Thompson PA, Lévy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175(3):462–466. doi:https://doi.org/10.1111/bjh.14324
- Khalid S, Yasar S, Khalid A, et al. Management of atrial fibrillation in patients on ibrutinib: a Cleveland clinic experience. Cureus. 2018. doi:https://doi.org/10.7759/cureus.2701
- Vrontikis A, Carey J, Gilreath JA, et al. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology (Williston Park). 2016;30(11):970-4, 980-1, C3.
- Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol 2018;4(11):1553–1568. doi:https://doi.org/10.1001/jamaoncol.2018.2706
- Roeker LE, Sarraf Yazdy M, Rhodes J, et al. Hypertension in patients treated with ibrutinib for chronic lymphocytic leukemia. JAMA Netw Open. 2019;2(12):e1916326. doi:https://doi.org/10.1001/jamanetworkopen.2019.16326
- Kapelios CJ, Bonou MS, Diamantopoulos P, et al. Ibrutinib-related atrial fibrillation: therapeutic challenges. J Oncol Pharm Pract. 2019;25(5):1258–1260. doi:https://doi.org/10.1177/1078155218785983
- Lane DA, Lip GYH. Use of the CHA2DS2-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860–865. doi:https://doi.org/10.1161/CIRCULATIONAHA.111.060061
- González-Correa JA, De La Cruz JP. Triflusal: an antiplatelet drug with a neuroprotective effect? Cardiovasc Drug Rev. 2006;24(1):11–24. doi:https://doi.org/10.1111/j.1527-3466.2006.00011.x
- Rogers KA, Mousa L, Zhao Q, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019. doi:https://doi.org/10.1038/s41375-019-0481-1
- Ball S, Das A, Vutthikraivit W, et al. Risk of infection associated with ibrutinib in patients with B-cell malignancies: A Systematic review and meta-Analysis of Randomized Controlled trials. Clin Lymphoma Myeloma Leuk. 2019. doi:https://doi.org/10.1016/j.clml.2019.10.004
- Arthurs B, Wunderle K, Hsu M, et al. Invasive aspergillosis related to ibrutinib therapy for chronic lymphocytic leukemia. Respir Med Case Reports. 2017;21:27–29. doi:https://doi.org/10.1016/j.rmcr.2017.03.011
- Tillman BF, Pauff JM, Satyanarayana G, et al. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 2018;100(4):325–334. doi:https://doi.org/10.1111/ejh.13020
- Hsiehchen D, Arasaratnam R, Raj K, et al. Ibrutinib use complicated by progressive multifocal leukoencephalopathy. Oncol. 2018;95(5):319–322. doi:https://doi.org/10.1159/000490617.
- EMA. Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg) (CPMP/BPWG/143744/2011 Rev.5). Guideline. 2018: 44. doi:CPMP/BPWG/143744/2011 Rev.1
- Narbutas Š, York K, Stein BD, et al. Overview on patient centricity in cancer care. Front Pharmacol. 2017;8(OCT):1–12. doi:https://doi.org/10.3389/fphar.2017.00698
- Langerbeins P, Bahlo J, Rhein C, et al. Ibrutinib versus placebo in patients with asymptomatic, treatment-naïve early stage CLL: primary endpoint results of the phase 3 double-blind randomized CLL12 TRIAL. Hematol Oncol. 2019;37:38–40. doi:https://doi.org/10.1002/hon.7_2629
- Shanafelt TD, Wang X V, Kay NE, et al. Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi:https://doi.org/10.1056/nejmoa1817073
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. doi:https://doi.org/10.1016/S1470-2045(18)30788-5
- Ahn IE, Farooqui MZH, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 2018;131(21):2357–2366. doi:https://doi.org/10.1182/blood-2017-12-820910
- Abrisqueta P, Loscertales J, Terol MJ, et al. Real-world characteristics and outcome of patients treated with single-agent ibrutinib for chronic lymphocytic leukemia in Spain (IBRORS-LLC study). Clin Lymphoma Myeloma Leuk. 2021. doi:https://doi.org/10.1016/j.clml.2021.07.022
- Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: Updated safety and efficacy results. Blood. 2015;126(6):739–745. doi:https://doi.org/10.1182/blood-2015-03-635326
- Rule S, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus: 3-year follow-up of patients with previously treated mantle cell lymphoma from the phase 3, international, randomized, open-label RAY study. Leukemia. 2018;32(8):1799–1803. doi:https://doi.org/10.1038/s41375-018-0023-2
- Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 Trial of Ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018;378(25):2399–2410. doi:https://doi.org/10.1056/nejmoa1802917
- Treon SP, Meid K, Gustine J, et al. Long-Term follow-Up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström macroglobulinemia. J Clin Oncol. 2021;39(6):565–575. doi:https://doi.org/10.1200/JCO.20.00555