ABSTRACT
Objective
Acute myeloid leukemia (AML) is a form of primary acute leukemia with high mortality. Our previous study demonstrated that miR-149-3p was down-regulated in chemoresistant acute leukemia cells. However, the biological function of miR-149-3p in AML needs to be further explored.
Methods
Herein, the expression of miR-149-3p was overexpressed/silenced in U-937 human AML cells via transfection with miR-149-3p agomir/antagomir. The effect of miR-149-3p on U-937-induced tumor growth was investigated using a xenograft nude mouse model.
Results
The results showed that miR-149-3p overexpression inhibited the proliferation and increased the apoptosis of U-937 cells. In addition, miR-149-3p suppressed epithelial–mesenchymal transition in U-937 cells, as demonstrated by the miR-149-3p agomir-induced increase in E-cadherin expression and decrease in vimentin expression. The in vivo experiments demonstrated that miR-149-3p suppressed tumor progression.
Conclusion
In conclusion, the findings revealed the association of miR-149-3p with the development of AML and suggest that miR-149-3p is a potential therapeutic candidate for AML.
Introduction
Acute leukemia is a heterogeneous type of malignant disorder defined by changes in mechanisms of differentiation, self-renewal, and proliferation in hematopoietic progenitor cells committed to the lymphoid or myeloid [Citation1]. Acute myeloid leukemia (AML) is a form of primary acute leukemia in adults characterized by the cumulation of malignant precursors of the myeloid lineage in the peripheral blood and bone marrow due to the loss of hematopoietic progenitor cells’ ability to differentiate into normal hematopoietic cells [Citation2–4]. In 2015 alone, an determined 27080 new case of acute leukemia were diagnosed in the United States, with over 76% of the cases being AML [Citation5]. The morbidity of AML increases with age, from ∼1.3 in every 10000 patients less than 65 years old to 12.2 in every 10000 patients over 65 years old [Citation6]. Despite decades of effort in clinical studies, the mainstream AML treatment is limited to chemotherapy, such as anthracyclines like daunorubicin, and the prognosis remains poor due to relapse and drug resistance [Citation7–10]. Targeted treatments development provides promise for more effective therapies with reduced side effects [Citation11,Citation12].
MicroRNAs (miRNAs) are small endogenous RNAs with 19–25 nucleotides that modulate gene expression post-transcriptionally [Citation13]. They are involved in the regulation of cellular processes such as apoptosis, proliferation, and epithelial–mesenchymal transition (EMT) [Citation14,Citation15]. The emergence of miRNAs has been one of the most decisive developments in tumor biology over the past decade and has provided new therapeutic and diagnostic opportunities for cancer [Citation16]. Accumulating studies have shown that miRNAs are related to the initiation and development of leukemia and act as promise therapeutic candidates in AML therapy [Citation17,Citation18]. For example, Vandewalle et al. found that miR-21-5p and miR-15a-5p were up-regulated in chemoresistant AML patients and contributed to chemoresistance in AML by targeting ARL2, BTG2, and PDCD4 [Citation19]. We previously demonstrated that miR-149-3p was down-regulated in chemoresistant acute leukemia cells, indicating that it may play an important function in chemotherapy failure [Citation20]. However, the biological function of miR-149-3p in AML needs further exploration.
To investigate the effect of miR-149-3p in AML, the expression of miR-149-3p was overexpressed or silenced in U-937 human AML cells via transfection with miR-149-3p agomirs or antagomirs, respectively. Cell proliferation and apoptosis were evaluated, and the effect of miR-149-3p on U-937-induced tumor growth was investigated in vivo.
Materials and methods
Cell culture and transfection
The human AML cell line U-937, HL-60 (human promyelocytic leukemia cells) (supplied by Shanghai Institutes for Biological Sciences, Chinese Academy of Science) was grown in RPMI-1640 medium and IMDM medium (Hyclone), respectively. Supplemented with 10% fetal bovine serum (Gibco) in an atmosphere of 95% air and 5% CO2. After the confluence reached 80–90%, 5 × 105 cells were suspended in 1.5 ml of RPMI-1640 or IMDM and seeded into each well of a 6-well plate. Both cells were transfected with miR-149-3p agomirs (miR-agomir), miR-149-3p antagomirs (miR-antagomir), or their corresponding negative control (NC) using Lipofectamine2000 (Invitrogen). After 24 h of transfection, the transfection efficiency was evaluated using quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Cell counting kit-8
Cell counting kit-8 (CCK-8) was performed to evaluate cell proliferation after transfection. Harvested transfected U-937 cells and HL-60 were grown in 96-well plates (3 × 103 cells in 100 μl of medium per well) and maintained at 37 °C with 5% CO2 for 24, 48, and 72 h. After 10 μl of CCK-8 solution (Solarbio) was added, the cells were further cultured for 4 h and the optical density was measured at 450 nm using an AMR-100 apparatus (Leica).
Hoechst 33258 staining
5 × 105 transfected cells were fixed in 0.5 ml of fixative for 30 min and washed twice with phosphate-buffered saline (PBS). The cells were resuspended in 50 μl of PBS, dropped onto a glass slide, and stained with 0.5 ml of Hoechst 33258 staining solution (Solarbio) for 5 min. After coating with antifade solution, the glass slide was viewed using an inverted fluorescence microscope (Leica) at 200× amplification.
Flow cytometry
1 × 106 transfected cells were resuspended in medium and centrifuged at 400×g, 4°C for 5 min. The cells were then resuspended in 200 μl of PBS and stained with 10 μl of Annexin V- fluorescein isothiocyanate (BD) and 10 μl of propidium iodide (BD) in the dark for 30 min. After adding 300 μl of PBS, flow cytometry was performed using a NovoCyte apparatus (ACEA Biosciences).
qRT-PCR
The transfection efficiency and mRNA expression of apoptosis-related genes in transfected U-937 cells were detected using qRT-PCR. Total RNA was extracted using Trizol (Ambion) and dissolved in DNase/RNase-free water (Solarbio). The collected RNA was reverse-transcribed into cDNA and amplified. The primary sequences are as follows: survivin forward, 5’-ACCACCGCATCTCTAC-3’, reverse, 5’-GGCTCTTTCTCTGTCCA3’; caspase 3 forward, 5’-GGTTCATCCAGTCGCTTT-3’, reverse, 5’-ATTCTGTTGCCACCTTTC-3’; Bcl-2 forward, 5’- CTGGTGGACAACATCGC-3’, reverse, 5’- GGAGAAATCAAACAGAGGC-3’; Bax, forward, 5’-TTCAGGGGATGATTGCC-3’, reverse, 5’-GCCTTGAGCACCAGTTTG-3’; GAPDH forward, 5’-GGGAAACTGTGGCGTGAT-3’, reverse, 5’-GAGTGGGTGTCGCTGTTGA-3’. GAPDH served as an endogenous control.
Western blot
Total protein was extracted from transfected U-937 cells using radioimmunoprecipitation assay lysis buffer (Solarbio) and quantified using a bicinchoninic acid kit (Solarbio). 20 μg of extracted protein were separated and transferred onto polyvinylidene fluoride membranes (Millipore). The membranes were blocked and incubated for 1 h with primary antibodies against survivin (Abcam), caspase 3 (Bioswamp), b-cell lymphoma-2 (Bcl-2) (Bioswamp), bcl-2 associated x protein (Bax) (Bioswamp), E-cadherin (Bioswamp), vimentin (Bioswamp), and GAPDH (Bioswamp), Then, the membranes were incubated with goat anti-rabbit IgG (Bioswamp) secondary antibody for 1 h. GAPDH served as an endogenous control.
Tumor xenograft in nude mice
Forty female specific-pathogen-free BALB/c nude mice aged 4–6 weeks (gifted from Changzhou Kavens laboratory Animal Co. Ltd.) were maintained at 22–26 °C with 50–60% relative humidity. After three days of adaptive feeding with free access to water and food, all mice were subcutaneously injected with 100 μl of U-937 cells (1 × 107 cell/ml) at the right axilla. When the tumor was clearly identifiable, tumor volume was measured every two days using the following formula: tumor volume (mm3) = length × width2/2 [Citation21]. The tumorigenesis rate was 75%. When the volume reached approximately 100 mm3, the nude mice were randomly divided into five groups (n = 6 per group): Control, miR-149-3p agomir (miR-agomir), miR-149-3p agomir negative control (Agomir-NC), miR-149-3p antagomir (miR-antagomir), and miR-149-3p antagomir negative control (Antagomir-NC). The mice in miR-agomir, Agomir-NC, miR-antagomir, and Antagomir-NC groups received multisite intratumoral injection of 30 μl of the respective substance at a concentration of 5 nmol (once every three days, four times). Mice in the control group received equal-volume saline treatment. At the end of the experimental period, the mice were sacrificed by an overdose of pentobarbital sodium (100 mg/kg). The tumors were removed, weighted, and fixed in 4% paraformaldehyde.
Hematoxylin–eosin (HE) staining
Pathological changes in the tumors were evaluated by HE staining. After embedding and slicing, the sections were dewaxed and stained with hematoxylin (Solarbio) for 3 min and eosin solution for 3 min (Solarbio). Pathological changes were assessed using a DM1000 apparatus (Leica, Germany).
Immunohistochemical staining
The protein expression of Ki-67 in tumor tissues was examined by immunohistochemistry. After embedding and slicing, tissue sections were dewaxed and hydrated before antigen retrieval using citrate buffer. The sections were blocked in 3% H2O2 for 15 min and immersed in 10% goat serum for 20 min. They were incubated with Ki-67 primary antibody (Bioswamp) overnight and MaxVisionTM HRP-Polymer anti-Mouse/Rabbit IHC secondary antibody (Fuzhou Maixin Biotechnology Development Co. LTD) for 45 min at room temperature. The samples were then visualized using diaminobenzidine (Solarbio) and counterstained using hematoxylin (Solarbio). Thereafter, the sections were observed using a microscope (Leica, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Statistical differences among data were analyzed using one-way analysis of variance followed by Tukey’s tests. *P < 0.05 was considered to be statistically significant.
Results
MiR-149-3p suppresses U-937 and HL-60 cell proliferation
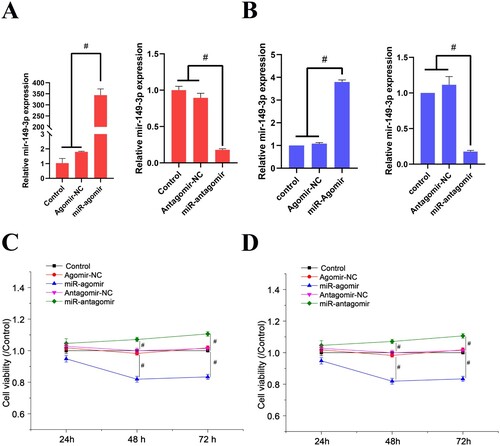
The transfection efficiency of miR-149-3p agomirs and antagomirs was evaluated using qRT-PCR. A demonstrates that miR-149-3p agomirs significantly increased miR-149-3p expression in U-937 cells, while miR-149-3p antagomirs significantly attenuated miR-149-3p expression in U-937 cells. The trend of miR-149-3p changes in HL-60 cells was similar to this (B). Meanwhile, the negative control of miR-149-3p agomirs and antagomirs showed no effect on miR-149-3p expression in U-937 and HL-60 cells. After 24, 48, and 72 h of transfection, the cell viability was detected using CCK-8 assay. The results revealed that miR-149-3p agomirs inhibited cell viability whereas miR-149-3p antagomirs increased it, indicating that miR-149-3p suppressed the proliferation of U-937 and HL-60 cells (C & D).
Figure 1. miR-149-3p attenuates proliferation of U-937 and HL-60 cells. (A) qRT-PCR was performed to detect the expression of miR-149-3p in U-937 cells after transfection. (B) qRT-PCR was performed to detect the expression of miR-149-3p in HL-60 cells after transfection. (C) CCK-8 assay was performed to detect the viability of U-937 cells after transfection. (D) CCK-8 assay was performed to detect the viability of HL-60 cells after transfection. Data represents the mean ± standard deviation. # p < 0.05.
MiR-149-3p promotes apoptosis and suppresses EMT in U-937 cells and HL-60 cells
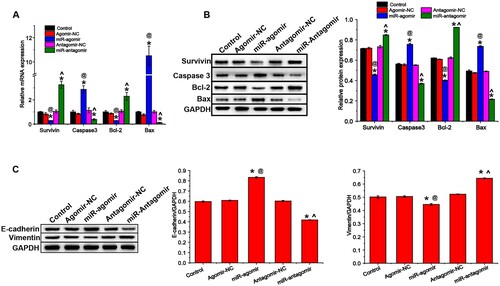
Apoptosis was assessed by Hoechst 33258 staining and flow cytometry. Hoechst results showed that miR-149-3p agomirs enhanced the apoptosis of U-937 cells, as demonstrated by the increase in the number of cells with bright blue staining (white arrow) and the percentage of apoptotic cells, respectively (A). The flow cytometry results were similar to Hoechst 33258 staining, miR-149-3p agomirs significantly increased apoptosis in U-937 and HL-60 cells (B & C). Then, the mRNA and protein expression of apoptosis-related factors were evaluated by qRT-PCR and western blot, respectively. miR-149-3p agomirs increased the expression of pro-apoptotic indicators (caspase 3 and Bax, A) and decreased that of anti-apoptotic indicators (survivin and Bcl-2, B). The results of qRT-PCR and western blot are consistent with those of Hoechst 33258 staining and flow cytometry. In addition, the expression of EMT-related proteins in transfected U-937 cells was detected by western blot. miR-149-3p agomirs increased the expression of E-cadherin and decreased that of vimentin, whereas miR-149-3p antagomirs showed the opposite results (C).
Figure 2. miR-149-3p promotes apoptosis of U-937 and HL-60 cells. (A) Hoechst 33258 staining was performed to examine the apoptosis of U-937 cells after transfection. (B) Flow cytometry was performed to detect apoptosis of U-937 cells after transfection. (C) Flow cytometry was performed to detect apoptosis of HL-60 cells after transfection. Data represents the mean ± standard deviation. * p < 0.05 vs. control; @ p < 0.05 vs. Agomir-NC; ^ p < 0.05 vs. Antagomir-NC.
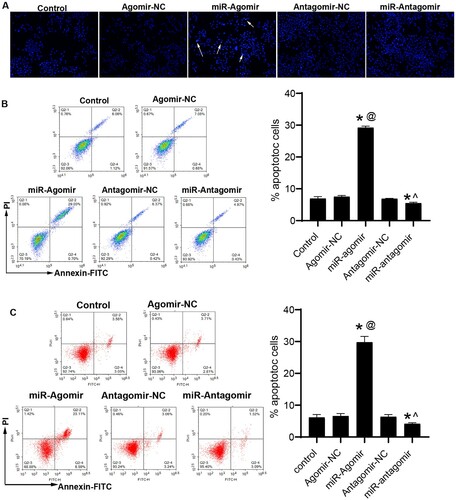
Figure 3. miR-149-3p regulates expression of proteins associated with apoptosis and EMT in U-937 cells. (A) qRT-PCR was performed to evaluate the mRNA expression of apoptosis-related indicators. Western blot was performed to evaluate the expression of proteins associated with (B) apoptosis and (C) EMT. Data represents the mean ± standard deviation. * p < 0.05 vs. control; @ p < 0.05 vs. Agomir-NC; ^ p < 0.05 vs. Antagomir-NC.
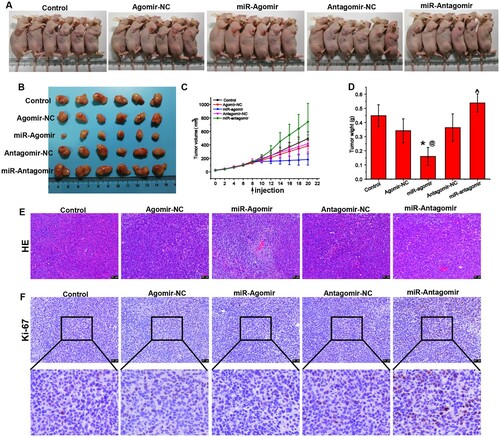
MiR-149-3p suppresses U-937-induced tumor growth
To investigate the effect of miR-149-3p on lymphoma in vivo, we constructed a xenograft mouse model via the injection of U-937 cells. The mice were treated with miR-149-3p agomirs/antagomirs or their corresponding negative controls. The results showed that miR-149-3p agomirs suppressed tumor growth, while miR-149-3p antagomir aggravated tumor growth (A-D). HE detection of the pathological morphology of the tumors indicated that miR-149-3p agomirs attenuated tumor progression ( E). In addition, the expression of Ki-67, a tumor proliferation marker [Citation22], in tumor tissues was detected using immunohistochemical staining. The results indicated that Ki-67 expression was decreased by miR-149-3p agomirs but increased by miR-149-3p antagomirs. Collectedly, the results indicated that miR-149-3p is involved in regulating the growth of tumors induced by U-937 cells.
Figure 4. miR-149-3p suppresses U-937-induced tumor growth. (A) Representative photos of tumor-bearing mice injected with U-937 cells and treated with miR-antagomirs/antagomirs. (B) Representative photos of tumors extracted from the mice. (C) Growth curve of tumor xenografts in mice. (D) Tumor weight on day 11 after treatment with miR-antagomirs/antagomirs. (E) HE staining of tumor tissue. (F) Immunohistochemical staining of Ki-67 protein expression in tumor tissue. Data represents the mean ± standard deviation. * p < 0.05 vs. control; @ p < 0.05 vs. Agomir-NC; ^ p < 0.05 vs. Antagomir-NC.
Discussion
MiR-149-3p is a new type of tumor-related factor, which delays the development of tumors by targeting and regulating certain factors in the body. In this work, we investigated the effect of miR-149-3p on AML cell proliferation and the expression of various marker proteins and genes, and overall described the effect of miR-149-3p on AML cell apoptosis. Yang et al. found that miR-149-3p inhibited the invasion, migration, and proliferation of bladder cancer cells by targeting S100A4 [Citation23]. Si et al. suggested that miR-149-3p overexpression promoted pancreatic cancer cell apoptosis by suppressing the level of Bcl-2, enhancing those of Bax and cleaved caspase 3/9, and contributing to cytochrome c release, which were associated with miR-149-3p-mediated inactivation of Akt1 signaling [Citation24]. Cao et al. demonstrated that miR-149-3p inhibited cell proliferation and induced apoptosis by targeting Wnt-1, thereby preventing the initiation and progression of gastric tumors [Citation25]. Yao et al. showed that miR-149-3p enhanced the apoptosis and suppressed the proliferation, migration, and invasion of chordoma cells in vitro and impaired chordoma tumorigenesis in vivo by downregulating Smad3 [Citation26]. Okato et al. revealed that miR-149 decreased the migration and invasion of renal cell carcinoma cells by targeting FOXM1 [Citation27].
This study indicated that miR-149-3p overexpression inhibited the proliferation of AML cells. In addition, miR-149-3p promoted U-937 cell apoptosis by decreasing the levels of surviving and Bcl-2 and increasing those of caspase 3 and Bax, which agrees with the results of previous studies [Citation24]. Apoptosis can be classified as either endogenous or exogenous [Citation28]. The main feature of the difference is that apoptosis caspases are activated in different ways. Exogenous apoptosis activates intracellular caspases through extracellular signals, while endogenous apoptosis activates caspases through mitochondrial release of apoptotic enzyme activating factors. Endogenous apoptosis, also known as mitochondrial apoptosis, is identified by the release of caspase activators such as cytochrome c into the cytoplasm. This results in the activation of caspase 9 and the cleavage of downstream caspases such as caspase 3, in turn inducing apoptosis [Citation29]. The release of cytochrome c is associated with the increased permeabilization of the outer mitochondrial membrane caused by the upregulation of Bax and downregulation of Bcl-2 [Citation30]. All indications suggest that miR-149-3p overexpression-induced apoptosis in U-937 cells and HL-60 cells may occur through endogenous apoptosis.
ETM plays an important role in embryonic development, chronic inflammation, tissue reconstruction, cancer metastasis and multiple fibrotic diseases. This study demonstrated that miR-149-3p inhibited EMT in U-937 cells by upregulating E-cadherin and downregulating vimentin, consistent with a previous study showing that miR-149-3p mimics suppressed EMT in colorectal cancer [Citation31]. EMT is a cellular process wherein epithelial cells transform into motile mesenchymal cells [Citation32]. In the process of EMT, junctions and apical-basal polarity are lost, prompting epithelial cells to undergo cytoskeletal rearrangement. This rearrangement leads to changes in the mechanotransduction of signaling pathways that affect cell shape and gene expression. In turn, the cells take on an invasive phenotype with increased motility [Citation32]. EMT is related to wound healing, embryogenesis, and malignant progression [Citation33], and the upregulation of vimentin, an EMT marker, has been shown to be correlated with poor clinical outcome in AML [Citation34]. Clinical experiments have also shown that the expression of E-cadherin, which acts as a prognostic biomarker in AML patients, was substantially downregulated in AML [Citation35].
Conclusions
In conclusion, the current study demonstrated that miR-149-3p inhibited cell proliferation and EMT in AML cells and promoted their apoptosis, suggesting that miR-149-3p is a potential therapeutic candidate for AML. However, only the first type of EMT in AML cells was studied in this research, and the specific mechanism of miR-149-3p-mediated inhibition of AML will be investigated in subsequent studies.
Supplemental Material
Download TIFF Image (242.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chung C, Ma H. Driving toward precision medicine for acute leukemias: are we there Yet? Pharmacotherapy. 2017;37(9):1052–1072.
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–1062.
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–1907.
- Shipley JLandButera JN. Acute myelogenous leukemia. Exp Hematol. 2009;37(6):649–658.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
- IandAbdul-Hay M DK. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441.
- Tamamyan G, Kadia T, Ravandi F, et al. Frontline treatment of acute myeloid leukemia in adults. Crit Rev Oncol Hematol. 2017;110:20–34.
- Nanbakhsh A, Visentin G, Olive D, et al. miR-181a modulates acute myeloid leukemia susceptibility to natural killer cells. Oncoimmunology. 2015;4(12):e996475.
- Zhou J, Ching YQ, Chng WJ. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: from molecular pathogenesis to therapeutic target. Oncotarget. 2015;6(8):5490–5500.
- Rautenberg C, Germing U, Haas R, et al. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. 2019;20(1):228.
- Hackl H, Astanina K, Wieser R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J Hematol Oncol. 2017;10(1):51.
- Zhou J, Goh BC, Albert DH, et al. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: from bench to bedside. J Hematol Oncol. 2009;2:33.
- Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207.
- Croce CMandCalin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7.
- White NM, Fatoohi E, Metias M, et al. Metastamirs: a stepping stone towards improved cancer management. Nat Rev Clin Oncol. 2011;8(2):75–84.
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469.
- Mardani R, Jafari Najaf Abadi MH, Motieian M, et al. MicroRNA in leukemia: tumor suppressors and oncogenes with prognostic potential. J Cell Physiol. 2019;234(6):8465–8486.
- Fernandes Q. MicroRNA: defining a new niche in leukemia. Blood Rev. 2017;31(3):129–138.
- Vandewalle V, Essaghir A, Bollaert E, et al. miR-15a-5p and miR-21-5p contribute to chemoresistance in cytogenetically normal acute myeloid leukaemia by targeting PDCD4, ARL2 and BTG2. J Cell Mol Med. 2020;25(1):575–585.
- Chen X, Guo Z, Fan S, et al. Integrating microRNA and mRNA expression in rapamycin-treated T-cell acute lymphoblastic leukemia. Pathol Res Pract. 2019;215(8):152494.
- Huang M, Huang Y, Guo J, et al. Pyrido[2, 3-d]pyrimidin-7(8H)-ones as new selective orally bioavailable threonine tyrosine kinase (TTK) inhibitors. Eur J Med Chem. 2020;211:113023.
- Menon SS, Guruvayoorappan C, Sakthivel KM, et al. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45.
- Yang D, Du G, Xu A, et al. Expression of miR-149-3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am J Cancer Res. 2017;7(11):2209–2219.
- Si L, Xu L, Yin L, et al. Potent effects of dioscin against pancreatic cancer via miR-149-3P-mediated inhibition of the Akt1 signalling pathway. Br J Pharmacol. 2017;174(7):553–568.
- Cao D, Jia Z, You L, et al. 18beta-glycyrrhetinic acid suppresses gastric cancer by activation of miR-149-3p-Wnt-1 signaling. Oncotarget. 2016;7(44):71960–71973.
- Yao J, Wu X. Upregulation Of miR-149-3p suppresses spinal chordoma malignancy by targeting Smad3. Onco Targets Ther. 2019;12:9987–9997.
- Okato A, Arai T, Yamada Y, et al. Dual strands of pre-miR-149 inhibit cancer cell migration and invasion through targeting FOXM1 in renal cell carcinoma. Int J Mol Sci. 2017;18(9):1969.
- Carmona-Gutierrez D, Eisenberg T, Buttner S, et al. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17(5):763–773.
- Wang L, Ma G, Zhang Y, et al. Effect of mitochondrial cytochrome c release and its redox state on the mitochondrial-dependent apoptotic cascade reaction and tenderization of yak meat during postmortem aging. Food Res Int. 2018;111:488–497.
- Adachi S, Cross AR, Babior BM, et al. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272(35):21878–21882.
- Chen D, Zhang M, Ruan J, et al. The long non-coding RNA HOXA11-AS promotes epithelial mesenchymal transition by sponging miR-149-3p in colorectal cancer. J Cancer. 2020;11(20):6050–6058.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196.
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84.
- Wu S, Du Y, Beckford J, et al. Upregulation of the EMT marker vimentin is associated with poor clinical outcome in acute myeloid leukemia. J Transl Med. 2018;16(1):170.
- Zhang TJ, Zhou JD, Ma JC, et al. CDH1 (E-cadherin) expression independently affects clinical outcome in acute myeloid leukemia with normal cytogenetics. Clin Chem Lab Med. 2017;55(1):123–131.
