ABSTRACT
Background
COVID-19 viral pandemic caused many mortalities in cancer patients especially those with hematological malignancies. The immunological response to COVID-19 infection is responsible for the outcome of cases whether mild, severe or critical.
Case presentation
Two cases presented with moderate COVID-19 viral infection, concomitant with acute myeloid leukemia and T acute lymphoblastic leukemia, respectively. Surprisingly, after the administration of COVID-19 supportive therapy, the cases showed disease remission after a follow-up period of 12 and 5 months, respectively. Additionally, the blast cells dropped to only 3% and 0% in the bone marrow aspirates of those two cases, respectively, after it was 30% in both cases at diagnosis.
Conclusion
The immune response that emerged against COVID-19 infection could potentially produce anti-tumor immunity in some patients, or the virus may act as an oncolytic virus. However, further investigations are required to explain this phenomenon, which may help in finding a possible new targeted therapy for these cases.
KEYWORDS:
Background
The novel COVID 19 viral pandemic caused a steep rise in infection-related mortality among cancer patients, which reached almost 30% [Citation1,Citation2]. This was even higher in cases with hematological malignancy, who are immunosuppressed and consequently more susceptible to COVID-19 infection and its complications [Citation3,Citation4,Citation5]. However, in the current study, we present two cases that went against this pattern.
Case 1
On 20 June 2020, a 63-year-old female patient presented with fever, dyspnea, wheezy chest and easy fatigability. She was diagnosed with COVID-19 infection by polymerase chain reaction (PCR) and underwent a routine diagnostic workup. The results came out: increased serum ferritin: 450 µg/L, increased C-reactive protein (CRP):100 mg/L, normal lactate dehydrogenase (LDH): 180 U/L, and normal D-dimer:324. The computed tomography (CT) showed bilateral ground-glass appearance (GGA). Additionally, her blood picture showed a total leucocytic count (TLC) of 12 × 103/UL, with 30% blast cells, Hemoglobin 7.3 g/dL, a platelet count of 82 × 103/UL with no lymphopenia. Clinically, the patient had moderate disease, with a blood oxygen level of 93 mmHg, she developed pneumonia but she did not need mechanical ventilation or ICU admission.
This prompted further investigations in the form of:
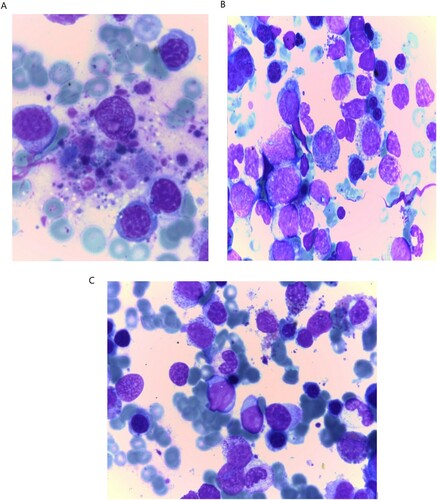
– Bone marrow aspirate (BMA) was normocellular with 53% blast cells, remarkable hemophagocytic features and dysplasia in the erythroid and myeloid series ((A,B)).
– Immunohistochemistry showed myeloid peroxidase positivity.
– Immunophenotyping gating on CD45 dim population was positive for MPO, CD13, CD33, MHC class II, CD64, CD34, CD11C, CD14 and CD117. Blast cells were diagnosed as myeloid with a monocytic differentiation phenotype.
– Cytogenetic findings were normal with no molecular findings as recurrent translocations, no mutation in FMS-like tyrosine kinase 3 internal tandem duplicate (FLT3-ITD) or nucleophosmin.
Figure 1. Features of dysplasia and blast cells in COVID-19 infected Acute myeloid leukemia (A): Hemophagocytic features (B): Blast cells with dysplastic features in myeloid and erythroid series (C) Reduction of blasts count after recovery from COVID-19 with eminent features of dysplasia in myeloid and erythroid series.
The patient was diagnosed with acute Myeloid Leukemia. However, her decision was to postpone her treatment protocol until she recovers from her viral infection, and she was given supportive therapy only. She received Fluconazole 150 mg three times daily, Azithromycin 500 mg daily and Prednisone 100 mg for five days. After two negative PCR nasopharyngeal swabs, and subsidence of the fever, the patient was discharged. However, her fatigability persisted, and surprisingly, on the 4th of August (five weeks) investigations were repeated to reveal:
– Complete blood picture showed hemoglobin concentration of 8.7 g/dL, TLC was 1.6 × 103/UL with no blast cells.
– BMA: blast count dropped to 3% with explicit dysplasia features in the trilineage with normal Karyotype ((C)).
All investigations were repeated three times and the patient was then diagnosed with Myelodysplastic syndrome refractory cytopenia with trilineage dysplasia.
Case 2
A male patient of 28 years old, previously diagnosed and treated as T-ALL since 2015. On 20 January 2021, the patient suffered from fever, headache, malaise, sore throat, dry cough, loss of smell and taste sensation. Nasopharyngeal swab confirmed COVID-19 infection. The patient had multiple cervical lymphadenopathy, high CRP: 28 mg/L, high serum ferritin level: 289 ng/mL, normal D-dimer: 125 ng/mL and normal LDH: 150U/L. The CT showed multiple bilateral GGA pneumonic patches mainly at the lower left lobe. The case showed moderate infection with the blood oxygen level of more than 93 mmHg. The TLC was 28 × 103/UL, with absolute lymphocytosis, and 30% blast cells, Hemoglobin concentration was 10.2 g/dL, and the platelet count was 150 × 103/UL. Relapse was suspected and accordingly, immunophenotyping was performed on the peripheral blood sample. It revealed increased expression of MHC class II, CD25, CD38, CD7, CD2, CD5, surface CD3 with CD4/CD8 ration 0.8 negative for TdT, CD34, CD1, CD99, CD56, CD16, CD33, CD13, MPO, CD117, CD19, CD22 and CD10. The patient decided to take COVID-19 supportive treatment and to repeat the investigations after recovery from COVID-19 infection. The patient was treated with Azithromycin 500 mg/day and Prednisone 40 mg daily for five days. Two weeks later, the PCR became negative for COVID-19. On 5 March 2021 (after 6 weeks), the cervical lymphadenopathy disappeared, and TLC decreased to 6.5 × 103/UL, no atypical cells were encountered, and the patient was monitored till the end of May 2021 with no relapse.
Both two cases were followed up till July 2021, and patients have not developed acute leukemia.
Discussion
The current COVID-19 pandemic posed a major challenge in the management of cancer patients, especially those with hematological malignancy. The co-existence of life-threatening conditions, including COVID-19 pneumonia and acute leukemia, makes the decision to use intensive chemotherapy exceedingly difficult. This could be explained by the myelosuppressive effect of the chemotherapy, in addition to the immunosuppressive effect of both chemotherapy and COVID-19 infection. Thus, haemato-oncologists have to weigh the risk as well as the benefits of the intensive potentially curable chemotherapy.
In the current two cases, patients were diagnosed as AML and relapsed ALL concomitantly with COVID-19 pneumonia, respectively. Patients refrained to start chemotherapy and received COVID-19 protocol with supportive measures. Approximately after one month, all evidence of AML and ALL started to disappear, and only the myelodysplastic features persist in the first case that was previously diagnosed as AML.
Our results of the two presented cases are in line with Challenor and Tucker case presentation, who also observed a remission condition in a Hodgkin Lymphoma patient. They reported that the lymphadenopathy subsided after COVID infection [Citation6]. This condition may be explained that the COVID-19 infection could potentially evoke an anti-tumor immune response through cross-reactivity of the virus-specific T cells with tumor antigens, or through non-specific activation of the natural killer cells by the inflammatory cytokines produced in response to viral infection [Citation6]. Similarly, Buckner et al. reported a spontaneous regression in a patient with diffuse large B-cell lymphoma co-existed with pneumonia and Clostridium difficile colitis. They proposed that this regression might be due to stimulation of the immune system with the co-existing pathogen [Citation7]. This leads to cross-reactivity of pathogen-specific T cells with tumor antigens, similar to the alloreactivity induced by viral-specific CD8 T cells that bind to human leukocyte antigen molecules [Citation8,Citation9]. These responses were used many times in the past in a trial performed by Starnes, for curing specific types of cancers like late-stage sarcoma using streptococcal infection, which was the base of Coley’s toxins theory [Citation10]. Another explanation could be that COVID-19 may act as an oncolytic virus, which is causing destruction of the tumor cells and release of the tumor-associated antigen (TAAs) from the tumor cells. These TAAs stimulate the immune system of the host against leukemia cells with cytotoxicity and apoptosis causing regression of the disease [Citation11].
In conclusion, there are some cases reported in the literature with hematological malignancies and showed spontaneous regression, especially if associated with a viral or bacterial infection. Therefore, extensive investigations are required to find an explanation for this regression. This may help in understanding the underlying immunological mechanisms associated with hematological malignancies, and consequently, finding a new potential therapeutic modality for those patients.
Acknowledgements
The study was approved by the ethical committee of the National Cancer Institute (NCI), Cairo University, according to the Helsinki principle. An informed consent was taken from the included patients in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Quteimat OM, Mustafa Amer A. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43(6):452–455.
- Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2021;72(2):340–350.
- Paul S, Rausch CR, Jain N, et al. The tissue issue: when to biopsy persistent or refractory disease in classic Hodgkin lymphoma. Acta Haematol. 2021;144(2):130–131.
- He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645.
- Malard F, Genthon A, Brissot E, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55(11):2180–2184.
- Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol. 2021;192(415):10–1111.
- Buckner TW, Dunphy C, Fedoriw YD, et al. Complete spontaneous remission of diffuse large B-cell lymphoma of the maxillary sinus after concurrent infections. Clin Lymphoma Myeloma Leuk. 2012 Dec;12(6):455–458.
- Burrows SR, Silins SL, Khanna R, et al. Cross-reactive memory T cells for Epstein–Barr virus augment the alloresponse to common human leukocyte antigens: degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur J Immunol. 1997;27:1726–1736.
- D’Orsogna LJ, Amir AL, Zoet YM, et al. New tools to monitor the impact of viral infection on the alloreactive t-cell repertoire. Tissue Antigens. 2009;74:290–297.
- Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–12.
- Lawler SE, Speranza M-C, Cho C-F, et al. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–849.
