ABSTRACT
Background
Immune thrombocytopenia (ITP) is a rare disease, characterized by increased platelet destruction/suboptimal platelet production, leading to thrombocytopenia and risk of severe bleeding events.
Methods
Interviews with 23 physicians and 12 payors, a survey with 113 physicians and validation using published data were used to define the current treatment paradigm and healthcare resource utilization and to determine the costs associated with managing acute bleeds in six European countries (Germany, Spain, France, Italy, Netherlands, UK). The study estimated a prevalence of 9 to 10 per 100,000 adults in 2020 across all six countries (disease severity split: 34% mild, 32% moderate, 33% severe (due to rounding up some values might not sum up to 100%).
Results
Physician feedback showed that most patients with ITP (60%) received first-line treatment or were monitored by their physician; ∼75% of patients relapsed within 3–4 months. Thrombopoietin-receptor agonists (TPO-RAs) and rituximab were used to achieve disease stabilization in patients who relapse; patients could switch to an alternative TPO-RA to control symptoms, manage side-effects or improve adherence. The costs of rescue therapies and hospital services (e.g. surgery and admissions) accounted for the majority of healthcare resources to manage bleeding events.
Conclusion
Physicians would welcome earlier use of TPO-RAs to help maintain long-term control of ITP bleeds and potentially reduce both hospitalization and therapy costs.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by increased platelet destruction and suboptimal platelet production resulting in low platelet counts. Diagnosis is based on a platelet count of <100 × 109/L and exclusion of other potential underlying causes of thrombocytopenia [Citation1]. Patients may exhibit a variety of symptoms, from non-life-threatening bleeding (e.g. easy or excessive bruising [purpura] or superficial bleeding of the mucosa/skin [petechiae]) to severe and/or fatal haemorrhage. Severe bleeding (non-intracerebral haemorrhage) with newly-diagnosed or chronic ITP is reported in 9.6% of adults and 20.2% of children [Citation2].
Published data on ITP epidemiology are limited, but a United Kingdom (UK) study among 840 adults representing over 20 million person-years (PYs) of follow-up found a higher incidence among women than men (4.5 vs. 3.2 per 100,000 PYs) and older age-groups (>65 years) [Citation3,Citation4]. Adults with ITP have a 1.5-fold higher mortality rate than the general population, with a 5-year mortality rate of 20%, largely due to increased cardiovascular disease, infection, bleeding and haematological cancer-specific mortalities [Citation5]. Complete remission is reported in 20–45% of patients within the first 6 months of diagnosis, although identifying spontaneous remissions beyond six months is more difficult due to the use of disease-modifying therapies [Citation2,Citation6].
Clinical guideline recommendations for first-line and acute treatment of ITP are similar across regions, but may vary for second- and third-line treatment due to consideration of onset/duration of effect and healthcare funding [Citation7–13]. First-line options include corticosteroids, intravenous immunoglobulin (IVIg) or anti-D immunoglobulin, aimed at decreasing autoantibody-mediated platelet clearance. Second/third line therapies include splenectomy – a potentially curative treatment for approximately two-thirds of patients – thrombopoietin receptor agonists (TPO-RAs) and off-label rituximab (anti-CD20 antibody) [Citation14,Citation15]. The management of adult ITP should be individually tailored, depending on bleeding symptoms, platelet count, bleeding risk due to lifestyle, and adverse effects.
While first-line treatment options are similar, most patients relapse, suggesting a significant unmet need for effective ITP therapy, and a thorough awareness of available options and their benefits is required for physicians to make informed decisions [Citation16]. We, therefore, conducted a qualitative observational study comprising interviews with haematologists and payers, and a survey of haematologists, across six European countries.
Aims of the study
The primary objectives of this real-world study were to understand the size of the ITP population in European countries, describe ITP patient characteristics, document the use of current therapies and drivers of treatment decisions and switching behaviour. The study also aimed (via a forecast model) to quantify the impact of current and future growth in the ITP population and on healthcare resource utilization.
Methods
Research was conducted during June and July 2020 across six European countries (France, Germany, Italy, the Netherlands [NL], Spain, and UK) via interviews with physicians and payers, supplemented by a separate physician survey and database searches. The interview guide and survey questions were designed specifically for this study.
Screening and recruitment
Participants identified by an external service vendor (M3 Global research) through literature searches, patient associations and professional networks, were invited to complete a screening questionnaire (English Language) (Table S1). Participant selection for the interviews was qualitative, based on responses to the screening questionnaire. Physicians were selected based on a number of treated patients (>10 each year) and ability to discuss epidemiology (qualitative feedback), with the intention to provide a balance of physicians working in university and general hospitals. Payers were selected based on previous experience with pricing and reimbursement for ITP therapies and ability to comment on the overall utilization of healthcare resources for ITP patients (qualitative feedback).
Screening questions for the online survey included medical specialty and proportion of treated adult patients (Table S1). Selection for participation was similar to the physician interview, i.e. number of treated patients and a balance of university/general hospital representation; other selection criteria were qualitative, including advanced or intermediate English language skills.
The study aimed to recruit four physicians and two payers per country (interviews), and 20 physicians per country (survey) to provide sufficiently representative information. Participants were invited via e-mail.
Conduct of interviews and survey
A panel of experienced researchers conducted the interviews and compiled the survey results. Researchers followed interview guides and conducted interviews by telephone, lasting 60–90 min. Participants were advised of the nature of the interview, and that they could withdraw at any point. Surveys were completed online in 15–20 min and responses to all questions were required for data to be included. Participants received a small honorarium based on time dedicated to the interview, or a fixed fee for survey completion.
Application of data
Quantitative data were derived from the physician survey or secondary sources (e.g. national treatment guidelines, published references and ITP/patient association websites); qualitative data were derived from secondary sources only; physician and payer interview responses were used to triangulate and verify all results (Tables S2 and S3).
Disease landscape
Compiled data were used to define ITP population size, the patient pathway and ITP management. ITP was characterized by the severity of platelet depletion (mild [platelet count >50 × 109/L], moderate [30–50 × 109/L] and severe [<30 × 109/L]); and duration of disease (new [<3 months], persistent (3–12 months] and chronic {>12 months]); and treatment was characterized by the line of therapy (first, second and third line).
Healthcare resource utilization
Treatment costs were calculated by deriving the average dosage of therapy per bleeding event, which was uniform across countries, and multiplying by the country-specific cost. Hospitalization costs were based on country-specific costs associated with specific diagnostic related group (DRG) codes [Citation17] for the main cost components for the treatment of ITP-related bleeds including hospital stays, diagnostic tests, imaging, healthcare staff time, rescue therapies, intensive care unit/ward bed occupancy, ambulance transport and surgery. Payer and physician interviews were used for cross-validation of cost data. All cost data collected were divided into two broad categories: cost of all therapies used, together with hospitalization, surgery, and human resources required for acute care.
Data analyses
Categorical variables were described by counts and proportions (average) of respondents. Missing data in the online survey were not explicitly addressed (e.g. imputed).
Future trends in the treatment landscape
A forecast model of ITP prevalence based on national population estimates was derived for two scenarios: (a) a base-case scenario, assuming current prescribing behaviour, and (b) an alternative scenario based on change in physicians’ preference over the next 5–10 years. Forecasting was designed using an independent modelling approach based on a patient flow methodology. No statistical analyses were conducted.
Ethical considerations
The research was conducted as a market research study conforming with current European Pharmaceutical Marketing Research Association guidelines and relevant legislation at the time of data collection [Citation18] and did not require ethics committee approval. Prior to agreeing to complete a questionnaire, all participants provided written informed consent to their involvement and understanding of the objectives. The survey was non-interventional and no identifiable patient records were accessed. Feedback could be shared among participants following the interviews; survey results were not available for further discussion.
Results
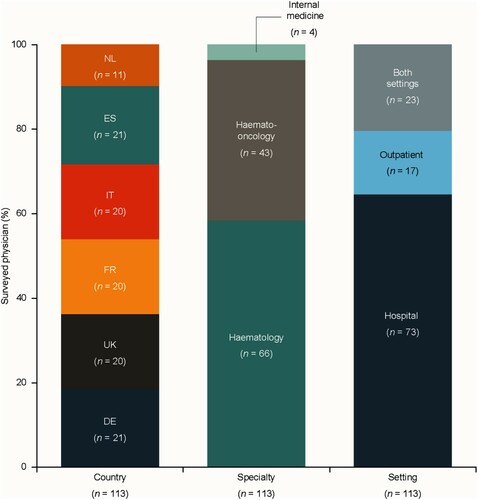
Interviews were conducted with 23 haematologists (France n = 4; Germany n = 4; Italy n = 5; NL n = 3; Spain n = 3; UK n = 4) and 12 payers (2 per country), supplemented by a separate bespoke survey of 113 physicians; distribution of survey participants by country, specialty and setting is shown in . Survey respondents managed 26–50 adult patients with ITP each year.
Figure 1. Geographic distribution, speciality and healthcare setting of survey respondents.
Source: physician survey, physician interviews and secondary data. DE: Germany; ES: Spain; FR: France; IT: Italy; NL: the Netherlands; UK: United Kingdom.
Size of ITP population
The estimated prevalence of ITP ranged from 9 to 10 per 100,000 adults, with the exception of Spain (12.6) and NL (18.1), supporting the European consensus of national ITP association estimated prevalence of 9.7 per 100,000 adults. This represents ∼27,000 adults with ITP across the six European countries based on aggregated population statistics. The survey indicated that numbers of patients with ITP were distributed proportionally across individual countries in this study based on overall population size, with 25% of all cases in Germany and 5% in NL.
Patient characteristics
Patients with ITP are characterized by duration of disease post-diagnosis or severity of thrombocytopenia. Although most patients present with bleeding and/or bruising symptoms, ∼40% of patients were indicated to be asymptomatic/in remission based on platelet count. Across all countries, the proportion of patients not in remission or asymptomatic were similar when stratified by disease severity: 34% mild, 32% moderate, and 33% severe.Footnote1
Treatment patterns
Overall, it was estimated that most patients with ITP receive ‘wait and see’ (29%) and first-line treatment (31%); second-line (24%) or third-line (16%) comprise the remainder. The distribution of ITP treatment across the disease severity groups indicated that during the first 3 months following diagnosis, most patients (61%) with mild ITP received a ‘wait and see’ approach and the remaining 39% receive first-line therapy (Figure S1). Patients with moderate ITP received ‘wait and see’, first- or second-line treatment approaches, while third-line therapy was reserved for those with severe ITP.
The ITP treatment paradigm was largely based on national guidelines in the six European countries surveyed (France, Germany, Italy, NL, Spain, and UK) (). After diagnosis, symptomatic patients are initially treated with corticosteroids due to their immediate effects. Patients with no improvements after 3–4 weeks can receive IVIg in combination with corticosteroids, while those with persistent low levels of platelets for >3 months receive second line pharmacotherapies (TPO-RAs, rituximab or fostamatinib) or undergo splenectomy. The data indicate that ∼75% of patients who receive first line therapy relapse within 3–4 months.
Figure 2. ITP treatment paradigm based on current national guidelines [Citation7,Citation9,Citation11–13].
IVIg: intravenous immunoglobulin; TPO-RA: thrombopoietin receptor agonist.
![Figure 2. ITP treatment paradigm based on current national guidelines [Citation7,Citation9,Citation11–13].IVIg: intravenous immunoglobulin; TPO-RA: thrombopoietin receptor agonist.](/cms/asset/97287b77-3c5d-4012-8af6-7e4e4ccc500c/yhem_a_1992945_f0002_oc.jpg)
TPO-RAs appear to be prescribed to 30–40% of patients across all countries, mainly as second- or third-line after failure of short-term therapies or splenectomy. While not licensed for ITP, rituximab was the most common alternative to TPO-RAs, with splenectomy mostly confined to third-line treatment. The duration of TPO-RA therapy varies, with some patients receiving treatment for several years. Rituximab was reported by 24–44% of physicians to be used second line for short-term/fast platelet count control before initiating chronic therapy with a TPO-RA. This approach may be preferred by some physicians to treat the underlying cause of ITP, rather than only focusing on boosting platelet count with TPO-RA.
The key drivers for TPO-RA use are their less invasive nature vs. splenectomy; superior efficacy over other therapies; and suitability for patients with severe disease. The main barriers to adoption of TPO-RAs were their suitability for mild/moderate patients, as well as the duration of therapy and side effects – especially in the mild/moderate patient segment, whose condition may not be sufficiently severe to warrant longer-term treatment. In the UK, access and reimbursement of TPO-RAs was reported to remain a barrier until national guidelines are updated to permit use as second line therapy.
Since the approval of several TPO-RAs, physicians reported switching between them for chronic patients following a lack of initial response to the first prescribed TPO-RA. A rapid fall in platelet counts following an initial response can also trigger the switch to a different TPO-RA, provided the fluctuation is not transient. Physicians described how TPO-RAs have different toxicity profiles, leading to rotation between products, whereby some patients may not tolerate subcutaneous formulations (e.g. romiplostim) due to skin infections and irritation, while others are vulnerable to adverse reactions to the oral formulations (e.g. eltrombopag) including mild gastrointestinal discomfort. Patient preference for a subcutaneous or oral formulation may also change over the course of treatment, leading physicians to switch TPO-RA to improve adherence to therapy.
Feedback indicated that physicians across Europe believe that failure on a TPO-RA does not reduce the success rate for a subsequent TPO-RA. Across all countries, 28–44% of patients received two TPO-RAs consecutively (Figure S2), while 17–40% of patients return to a TPO-RA regimen following an alternative (non-TPO-RA) therapy. In accordance with guidelines suggested by the American Society of Haematology [Citation15], some physicians reported an opportunity for earlier TPO-RA adoption in more severe disease, considering the response rate to be higher than existing therapies and that a greater rate of remission may be achieved by starting therapy earlier. Moreover, physicians expressed interest in replacing corticosteroids as a first-line therapy in elderly patients due to the lower potential for this group to tolerate the side effects.
Healthcare resource utilization
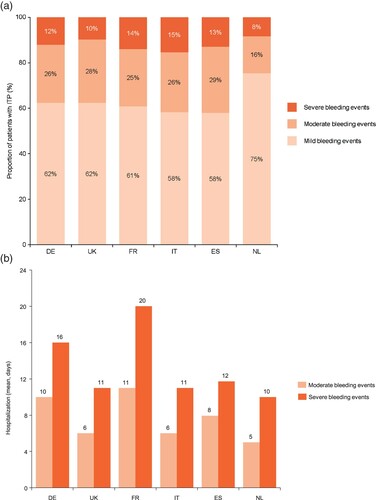
Patients with ITP may experience bleeding episodes requiring outpatient treatment or, occasionally hospitalization. Bleeding events in patients with moderate or severe ITP (around 3% and 5% per year, respectively) were more frequent than in those with mild ITP (around 1% per year). Acute bleeding events can be divided into three categories of severity: mild bleeding events, accounting for 58–75% of all bleeds requiring medical intervention, while moderate or severe bleeds account for 16–28% and 8–15% of all events, respectively ((a)). Feedback indicates that patients with moderate bleeding events are hospitalized for, on average, 6–11 days, while severe bleeds usually require 10–20 days of hospitalization ((b)). ITP-related bleeding events occur primarily pre-diagnosis, when patients are not on treatment; some bleeding events are also observed while on therapy.
Figure 3. Proportion of patients with mild, moderate or severe bleeding events and associated days of hospitalization by country.
Data may not sum to 100% due to rounding. Source: physician survey, physician interviews and secondary data. DE: Germany; ES: Spain; FR: France; IT: Italy; ITP: immune thrombocytopenia; NL: the Netherlands; UK: United Kingdom.
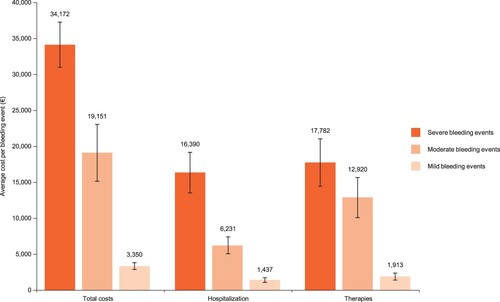
The management of ITP-related bleeding events appears to be standardized across the countries studied. In each country, costs for managing bleeds increase proportionately to the severity (Table S4), with severe, moderate and mild bleeds accounting for an average of €34,200, €19,200 and €3,400, respectively (). Hospitalization costs contribute equally with therapy costs for severe bleeds (approximately €16,000 and €18,000, respectively; ).
Future trends in the treatment landscape
Physicians indicated that the diagnosed prevalence of ITP will continue to grow (). The ITP patient population is expected to grow by ∼2.5% annually (p.a.) from ∼27,000 patients in 2020 to ∼35,000 patients by 2030, driven by population growth, low mortality rate, low remission rate with ongoing therapies, and adoption of chronic treatments over splenectomy (Figure S2). The increasing use of long-term treatment is a key driver for the projected acceleration in treated ITP population growth in the first 5 years (2020–2025), at 4.3% p.a. ((a)). The ‘wait and see’, second- and third line patient segments are expected to experience most growth compared with first line.
Figure 5. Forecast compound annual growth rate in ITP population by treatment line (a) and by ITP severity (b) over the next 5–10 years*.
*Due to rounding up some values might not sum up to 100%. Source: physician survey, physician survey and secondary data. CAGR%: compound annual growth rate; ITP: immune thrombocytopenia.
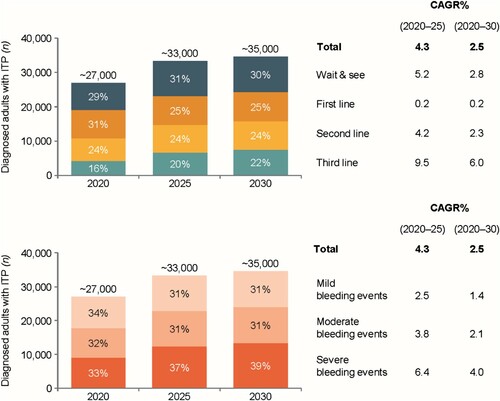
Figure 6. Forecast uptake of TPO-Ras by country over the next five years.
Data may not sum to 100% due to rounding. Source: physician survey and secondary data. DE: Germany; ES: Spain; FR: France; IT: Italy; ITP: immune thrombocytopenia; NL: the Netherlands; TPO-RA: thrombopoietin receptor agonist; UK: United Kingdom.
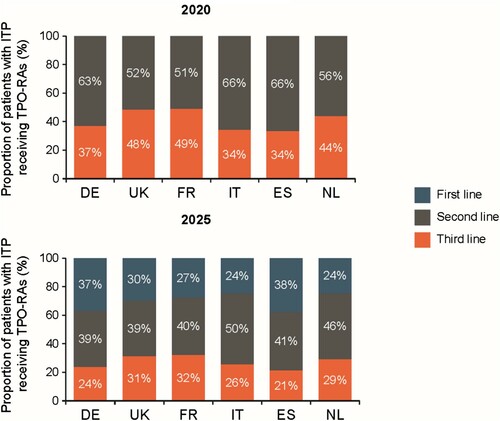
The forecast model suggests an overall increase in the proportion of patients receiving third-line therapy of ∼6.0% p.a. over 10 years, driven by use of TPO-RAs instead of splenectomy ((a)), and an annual 4% overall increase in patients with severe disease over the next 10 years ((b)). Over the next 5 years, the overall proportion of patients receiving TPO-RAs is forecast to increase, with the proportion receiving TPO-RAs as second- and third-line therapy declining, as their use in first-line treatment increases (). This should create a stable, first line patient cohort on long-term therapy.
Discussion
This multinational study investigated the epidemiology, patient characterization, and expected growth in chronic ITP. It also evaluated the treatment paradigm, key drivers, and use of current therapies, including TPO-RAs, and estimated the impact of the current standard of care on healthcare resource utilization as it relates to use of rescue therapies for bleeds. The strength of the study is that it reflects genuine treatment decisions and patterns from a real-world setting and provides insight into routine management of ITP.
The introduction of the TPO-RAs, romiplostim, eltrombopag and avatrombopag, heralded a paradigm shift in the treatment of ITP [Citation19]. Our study found that TPO-RAs are a popular choice in second- and third-line settings, while rituximab and other immunosuppressants (such as methylprednisolone) are sometimes prescribed to provide rapid control of platelet count before initiating a TPO-RA regimen for chronic treatment. Additionally, physicians may switch to an alternative TPO-RA following a rapid fall in platelet counts. Indeed, the benefits of switching between TPO-RAs has been reported [Citation20,Citation21], which may encourage physicians to adopt this approach.
While rituximab and splenectomy can provide short-term remission for ITP, long-term responses are not completely satisfactory [Citation19]; after 2–5 years, ∼60% have complete response with splenectomy and ∼20% treatment-free response with rituximab [Citation22,Citation23]. Adverse events with these interventions may be significant, including increased infection risk and thromboembolism after splenectomy, and rare cases of progressive multifocal leukoencephalopathy and increased infection rate after rituximab [Citation24]. Splenectomy was the least preferred choice across the countries and largely positioned as a third-line treatment option due to the emergence of newer therapeutic options [Citation14,Citation25].
Physicians suggested that the tolerability, superior efficacy, suitability for more severe disease, and less invasive nature vs. splenectomy, could position TPO-RAs into first-line treatment, but this would require ratification by regulatory and reimbursement agencies. Understanding how to choose between TPO-RAs, their dosing, switching between TPO-RAs, recognizing when discontinuation is appropriate, considering combination therapy in treatment-refractory patients, and managing side effects are essential factors guiding their optimal use.
A reported incidence rate for ITP of 1.6–3.9 per 100,000 patient-years, which increases with age and has a slight female preponderance [Citation1] is consistent with findings from our study. Previous ITP epidemiology studies relied on documentation of ITP with a specific ICD code to define diagnosed incidence. Our study indicates that the overall patient population will grow at ∼2.5% p.a. for the next 10 years, largely due to the incidence of ITP, low mortality rate and high remission rate with ongoing therapies, together with the underlying population growth (accounting for ∼0.5%). The population segment with severe disease is also expected to rise over the next 10 years, as well as an ongoing shift of patients into long-term therapies (primarily TPO–RAs).
Numerous hospital resources and rescue therapies are required to manage ITP-related bleeding events. Our study supported that moderate and severe bleeding events (accounting for around 40% of all bleeds) demand disproportionately more resource, and are estimated to cost up to €33,000 per episode across the countries surveyed. Physicians indicated that the earlier and more frequent use of TPO-RAs could lead to fewer bleeds, and lower associated costs.
The use of physicians and payers from different settings and six countries indicate the sample is likely to be representative of the overall management of patients with ITP across Europe. However, the sample size for the interviews in particular may be considered small and selection bias may have occurred; therefore, generalizability should be treated with caution. Further, the sample may not have been evenly distributed across regions and may have been weighted by physicians treating larger populations of patients with ITP. Self-reported information is susceptible to bias, and there may have been systematic errors caused by differences in the accuracy of the data provided by the participants. Moreover, in this survey, all data collected on ITP diagnosis, management and outcomes were based on subjective opinion without validation. It is also difficult to directly compare the survey results with those of other epidemiological studies, largely due to differences in methodology, e.g. differences in ITP definitions, and age groups.
Conclusions
Adoption of long-term therapies, together with low mortality rates and high remission rates with ongoing therapies, means that the ITP patient population will likely comprise greater proportions of patients with severe disease and undergoing long-term therapy over the next ten years. Therapy duration, tolerability, suitability for mild/moderate patients, and market access (reimbursement) all impact treatment decisions across Europe. However, both the early uptake and long-term treatment with effective and well-tolerated TPO-RAs have the potential to significantly reduce hospitalization and therapy costs associated with bleeding events in patients with ITP.
Supplemental Material
Download MS Word (146 KB)Acknowledgements
Medical writing/editorial support for this manuscript was provided by K. I. Johnson BSc MBPS, SRPharmS and Tyrone Daniel PhD, of Bioscript, Macclesfield, UK.
Disclosure statement
Edgar A. Pogna, Simon Middleton and Leah Ralph are the employees of L.E.K. Consulting, London, UK, who were contracted by Sobi to support the design and conduct this research. Jameel Nazir and Koo Wilson are employees of Sobi. Wojciech Jurczak has received advisory board fees from Sobi.
Additional information
Funding
Notes
1 Due to rounding up some values might not sum up to 100%.
References
- Kohli R, Chaturvedi S. Epidemiology and clinical manifestations of immune thrombocytopenia. Hamostaseologie. 2019;39(3):238–249.
- Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457–464.
- Abrahamson PE, Hall SA, Feudjo-Tepie M, et al. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 2009;83(2):83–89.
- Weycker D, Hanau A, Hatfield M, et al. Primary immune thrombocytopenia in US clinical practice: incidence and healthcare burden in first 12 months following diagnosis. J Med Econ. 2020;23(2):184–192.
- Frederiksen H, Maegbaek ML, Nørgaard M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2014;166(2):260–267.
- Sailer T, Lechner K, Panzer S, et al. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica. 2006;91(8):1041–1045.
- Deutsche Gesellschaft fuer Haematologie und medizinische Onkologie (DHGO). German ITP guidelines 2020. Available from: https://www.onkopedia.com/de/onkopedia/guidelines/immunthrombozytopenie-itp/@@guideline/html/index.html.
- General Haematology Task Force. British committee for standards in haematology. Br J Haematol. 2003;120:574–596.
- Italian National Academy of Medicine. ITP guideline 2001. Available from: http://www.ematologialasapienza.org/Download_file_sezione.asp?Chiave=&ID_sezione=170.
- Nederlandse Vereniging voor Hematologie. Richtlijn Immuun gemedieerde trombocytopenie 2013. Available from: https://hematologienederland.nl/wp-content/uploads/2020/01/ITP_richtlijn_2013__NVvH_.pdf.
- NICE. Treatment summary: Immune thrombocytopenic purpura 2019. Available from: https://bnf.nice.org.uk/treatment-summary/immune-thrombocytopenic-purpura.html.
- Protocole National de Diagnostic et de Soins. Purpura thrombopénique immunologique l’enfant et de l’adulte 2017. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2017-06/dir36/pnds-_purpura_thrombopenique_immunologique.pdf.
- Sociedad Española de Hematología y Hemoterapia. Documento de Consenso. Directrices de diagnóstico, tratamiento y seguimiento de la PTI 2011. Available from: http://www.sehh.es/documentos/40/Guia%20PTI.pdf.
- Khan AM, Mydra H, Nevarez A. Clinical practice updates in the management of immune thrombocytopenia. P T. 2017;42(12):756–763.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866.
- Onisâi M, Vlădăreanu AM, Spînu A, et al. Idiopathic thrombocytopenic purpura (ITP) – new era for an old disease. Rom J Intern Med. 2019;57(4):273–283.
- DRG Research Group. NHS National Tariff Workbook 2019/2020.
- EphRMA. European Pharmaceutical Market Research Association (EphMRA) Code of Conduct 2019. Association EPMR; 2019.
- Ghanima W, Cooper N, Rodeghiero F, et al. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6):1112–1123.
- Kuter DJ, Macahilig C, Grotzinger KM, et al. Treatment patterns and clinical outcomes in patients with chronic immune thrombocytopenia (ITP) switched to eltrombopag or romiplostim. Int J Hematol. 2015;101(3):255–263.
- Gonzalez-Porras JR, Godeau B, Carpenedo M. Switching thrombopoietin receptor agonist treatments in patients with primary immune thrombocytopenia. Ther Adv Hematol. 2019;10. DOI:2040620719837906
- Rodeghiero F. A critical appraisal of the evidence for the role of splenectomy in adults and children with ITP. Br J Haematol. 2018;181(2):183–195.
- Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–5995.
- Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: what do we know after 20 years of rituximab. Rev Med Virol. 2019;29(6):e2077. DOI:https://doi.org/10.1002/rmv.2077
- Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495–520.